<b>Cooley</b>, S. R., D. J. P. <b>Moore</b>, S. R. Alin, D. Butman, D. W. Clow, N. H. F. French, R. A. Feely, Z. I. Johnson, G. Keppel-Aleks, S. E. Lohrenz, I. B. Ocko, E. H. Shadwick, A. J. Sutton, C. S. Potter, Y. Takatsuka, A. P. Walker, and R. M. S. Yu, 2018: Chapter 17: Biogeochemical effects of rising atmospheric carbon dioxide. In Second State of the Carbon Cycle Report (SOCCR2): A Sustained Assessment Report [Cavallaro, N., G. Shrestha, R. Birdsey, M. A. Mayes, R. G. Najjar, S. C. Reed, P. Romero-Lankao, and Z. Zhu (eds.)]. U.S. Global Change Research Program, Washington, DC, USA, pp. 690-727, https://doi.org/10.7930/SOCCR2.2018.Ch17.
Biogeochemical Effects of Rising Atmospheric Carbon Dioxide
Over geological time (i.e., the last 500 million years), atmospheric CO2 levels have at times been well in excess of current CO2 concentrations (see Figure 17.2). However, human civilization developed during the last 10,000 years, a time when atmospheric CO2 was never higher than it is today (Augustin et al., 2004). Once humans began extensively altering the landscape and burning fossil fuels, atmospheric CO2 and CH4 began to rise rapidly and drive changes in atmospheric, terrestrial, and oceanic systems and processes (Olofsson and Hickler 2007).
Figure 17.2: Geological Context of Carbon Dioxide (CO2)
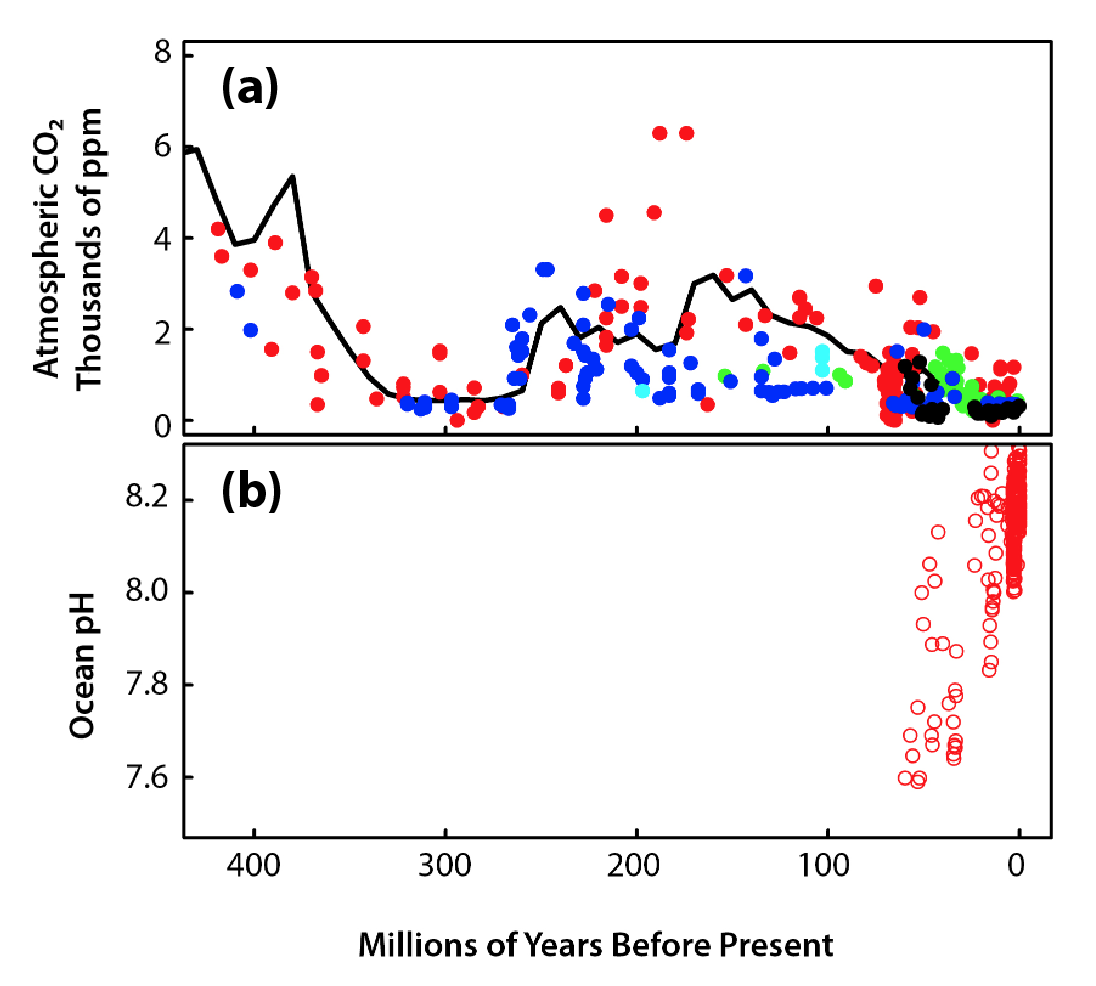
Changes in atmospheric CO2 changed Earth’s climate and ocean pH and altered the course of plant evolution. Atmospheric CO2 was likely higher than 5,000 parts per million (ppm) at times during the last 540 million years (Phanerozoic Eon) and declined to current levels during the last 25 million years (Doney and Schimel 2007; Royer 2006; see Figure 17.2). During this eon, periods of frequent glaciation events in Earth’s history are associated with CO2 concentrations below 1,000 ppm (Royer 2006). A strong decline of atmospheric CO2 during the Carboniferous Period (359 million years ago) is associated with the proliferation of land plants. Extensive burial of plants from this period resulted in the massive deposits of fossil fuels now being mined. Declining atmospheric CO2 concentrations at the Eocene-Oligocene boundary (34 million years ago) induced dynamic ice sheet formation over Antarctica and ultimately led to substantial cooling of global climate over the subsequent 10 million years (DeConto and Pollard 2003). During the Quaternary Period (last 1 million years), ice core records show that temperature increases of ~3°C were associated with CO2 increases of ~100 ppm (Petit et al., 1999). Recent analyses show that during the last deglaciation (from ~21,500 to ~11,500 years ago), observed increases in global temperature lagged behind observed increases in atmospheric CO2 (Shakun et al., 2012). The glacial-interglacial cycle in Earth’s climate during the Quaternary period is caused by a combination of changes in Earth’s orbit, atmospheric greenhouse gases, and ocean circulation (Rohling et al., 2018).
The evolution of different ways of performing photosynthesis has a strong influence on the non-climate consequences of rising CO2 on land. Fundamental to plant life on Earth, atmospheric CO2 concentrations and their dynamics over geological time have played an important role in the evolution of photosynthesis and the distribution of different vegetation types (Beerling et al., 2001; Monson and Collatz 2011). RUBISCO (ribulose-1, 5-bisphosphate carboxylase-oxygenase), the enzyme that catalyzes the transfer of atmospheric CO2 into plant sugars and biomass, evolved in early algae during a time of high CO2 at least 2.8 billion years ago (Doney and Schimel 2007), though perhaps much earlier (Allwood et al., 2006; Raven et al., 2012). Plants evolved different photosynthetic mechanisms and anatomies in response to the relatively low CO2 concentrations that persisted from about 300 million years ago, an environment which enabled C4 grasses (e.g., ancestors of maize, sugarcane, and sorghum) and the cactus family to dominate arid portions of the Earth because of their greater water-use efficiency and drought tolerance (Berner 1997; Osborne and Sack 2012; Pagani et al., 2005).
Prior geological eras also provide information about potential impacts of high atmospheric CO2 on ocean chemistry (Hönisch et al., 2012). Atmospheric CO2 dissolves in seawater and creates carbonic acid, which lowers pH and decreases the concentration of carbonate ions present in solution. The closest analogs to present conditions may be the Paleocene-Eocene Thermal Maximum (56 million years ago), Triassic-Jurassic mass extinction (~200 million years ago), and Permo-Triassic mass extinction (252.3 million years ago; Hönisch et al., 2012). All these events are associated with evidence of detrimental impacts on calcifying organisms including, in some instances, their extinction. However, definitively attributing negative effects on calcifiers to acidification is not possible because of other factors (e.g., ocean circulation, warming, oxygenation, and asteroid impacts) that may have co-occurred or contributed to the decline or demise of these organisms. Moreover, geochemical proxies indicating pH or ocean carbonate chemistry conditions, particularly for times before the Cretaceous Period (>65 million years ago), are limited and have large uncertainties.
Since the start of the Industrial Revolution, anthropogenic emissions have resulted in increased atmospheric CO2 concentrations detectable by changes in the ratio of 13C and 12C isotopes in the biosphere (Keeling 1979; Suess 1955). Fossil fuels have less of the 13C isotope because they are composed of dead plants and animals, and burning them changes the isotope ratio in the atmosphere. Isotopic studies indicate some of the carbon released from fossil sources becomes incorporated into all organisms, including those as diverse as trees (Suess 1955), marine fish (Fraile et al., 2016), and penguins (Hilton et al., 2006). The decrease in ocean pH since the start of the Industrial Revolution matches or exceeds the pH levels observed for the Quaternary glacial-interglacial period (Pelejero et al., 2010; Turley et al., 2006). Moreover, projected changes in ocean pH by 2100 well exceed those that occurred during the preindustrial period (Bijma et al., 2013; Turley et al., 2006). Recent global changes in upper ocean chemistry likely are occurring more rapidly than at any time over the past 300 million years (Doney et al., 2014; Hönisch et al., 2012). The rates and magnitude of change may soon move the ocean ecosystem into “uncharted territory,” with conditions unlike any that contemporary marine life have faced during their recent evolutionary history (Gattuso et al., 2015; Turley et al., 2006).
See Full Chapter & References