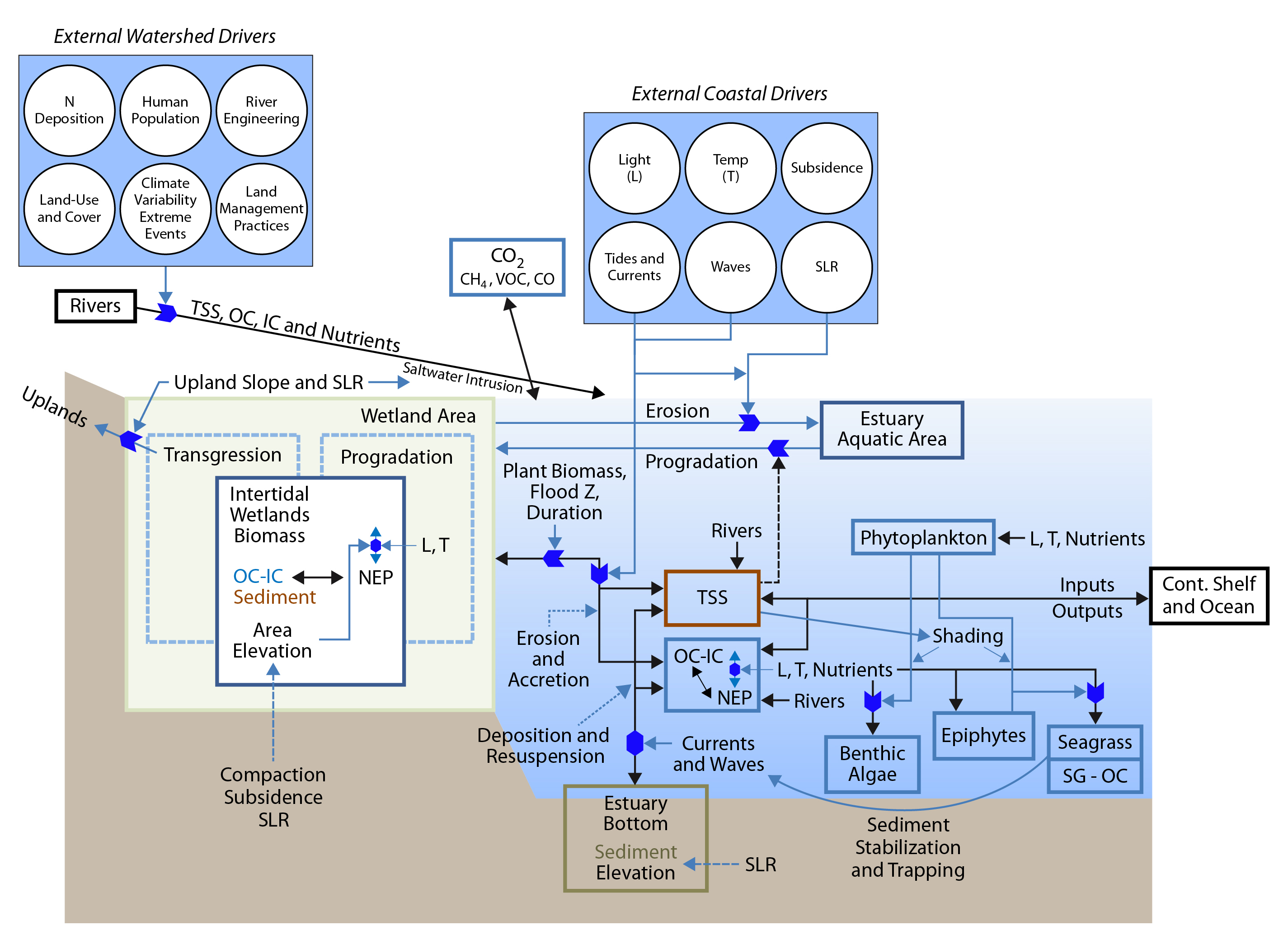
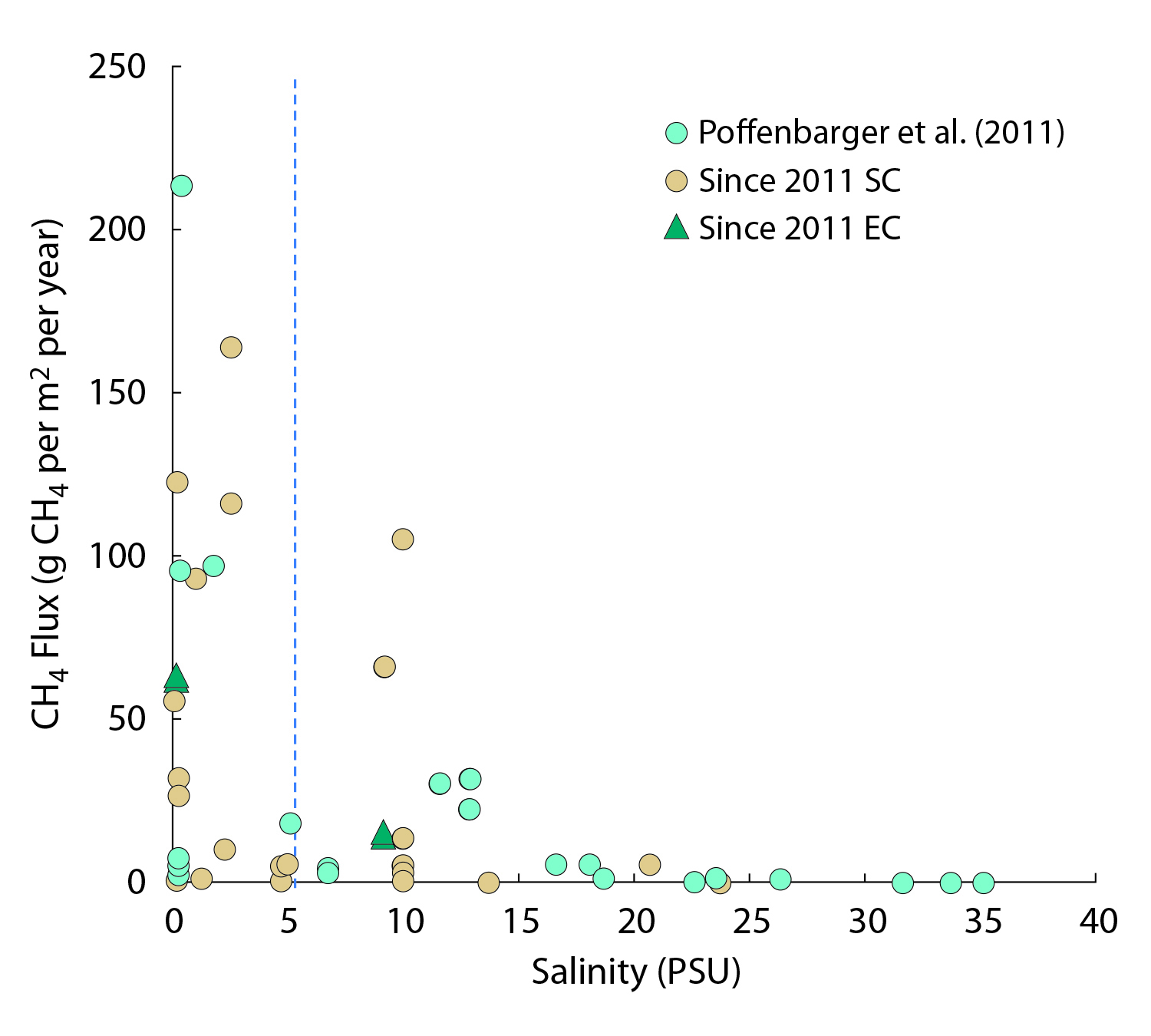
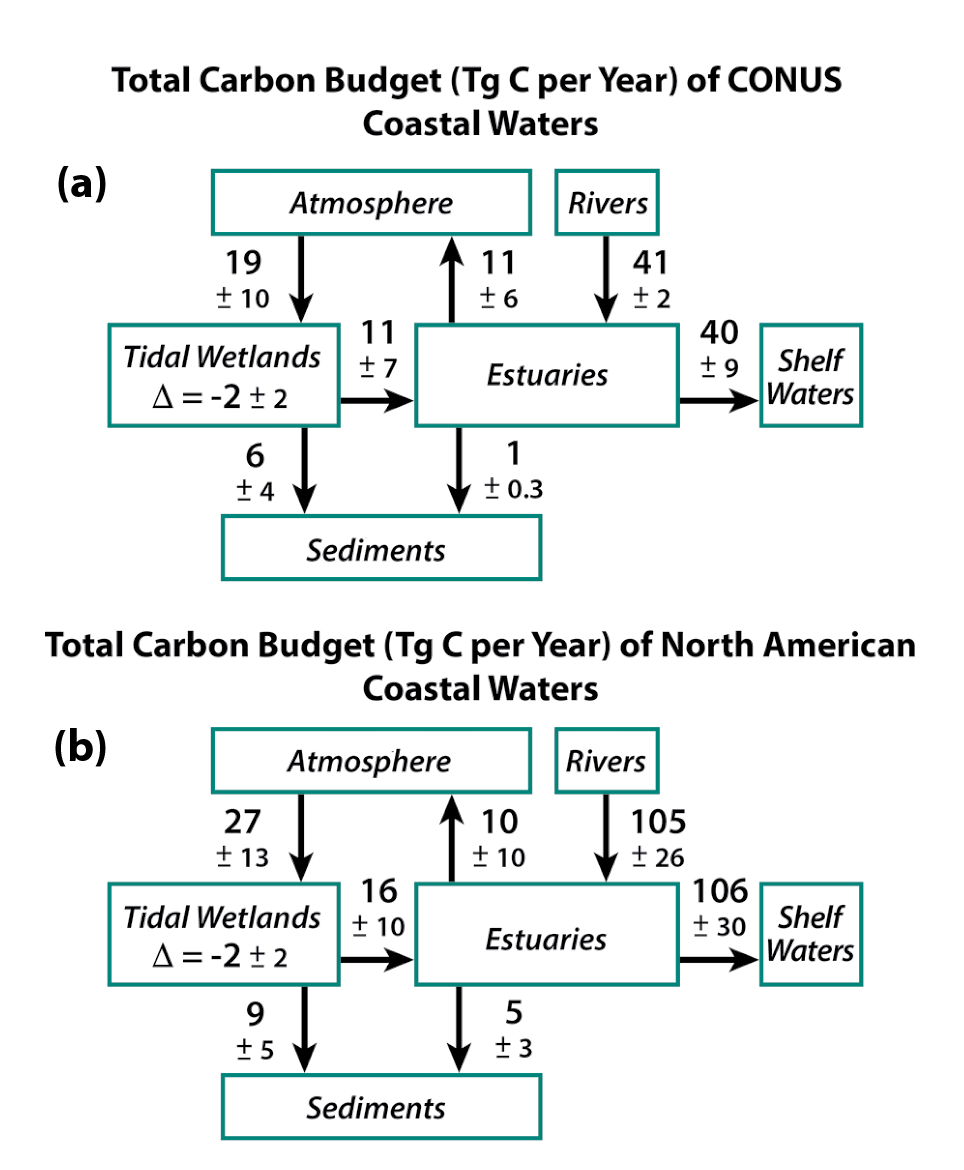
15.3.1 Atlantic Coast Estuaries and Tidal Wetlands
Estuaries of the North American Atlantic coast are the most extensive and diverse in structure and function within North America. Relatively shallow and driven primarily by landward influences, they are strongly influenced by freshwater flow and quality from rivers and groundwater. From boreal to subtropical latitudes, a wide range of biotic activity (e.g., photosynthesis and respiration) is seen from Nova Scotia to Florida.
Atlantic Coast Estuaries
South Atlantic Bight. The South Atlantic Bight (SAB: southern tip of Florida to Cape Hatteras, North Carolina) is a passive, western boundary current margin with broad shelf areas, extensive shoals, and a series of barrier islands, behind which are lagoons. Freshwater delivery in the SAB is through rivers that are nearly evenly located along the coast. These rivers carry high loads of dissolved organic carbon (DOC). Because of short transit times through the estuaries, much of the DOC is discharged onto the shelf, supporting respiration, net heterotrophy (Hopkinson 1985, 1988), and CO2 degassing on the inner-shelf regions (Jiang et al., 2013). Much is known about the export of organic matter from SAB watersheds. The SAB salt marshes are tremendous sinks of CO2 and organic carbon from uplands, whereas the estuarine waters are strong sources of CO2 to the atmosphere—sources that are largely supported by organic matter and dissolved inorganic matter (DIC) export from both wetland saltmarshes and from SAB watersheds (Wang and Cai 2004; Cai 2011; Herrmann et al., 2015; Hopkinson 1988).
Mid-Atlantic Bight and Gulf of Maine. The Mid-Atlantic Bight (MAB: Cape Hatteras, North Carolina, to Cape Cod, Massachusetts) and Gulf of Maine (GOM: Cape Cod to Nova Scotia) are characterized by large estuaries. Inorganic carbon from carbonate weathering and organic matter remineralization accounts for the majority of riverine carbon input to the MAB (Hossler and Bauer 2013; Moosdorf et al., 2011). Generally, aqueous organic matter concentrations are higher in southern MAB rivers and can be more than half the riverine carbon load to estuaries (Stets and Striegl 2012; Tian et al., 2015). Lateral exchange with wetlands is an important carbon input to MAB waters and has been linked to net heterotrophy and air-water CO2 efflux in narrow, marsh-dominated subestuaries (Baumann et al., 2015; Raymond et al., 2000; Wang et al., 2016). However, larger MAB estuaries can be seasonal or annual sinks for atmospheric CO2 because of stratification and high rates of internal production (Crosswell et al., 2014; Joesoef et al., 2015). Supporting this result, recent carbon budget studies have estimated that MAB estuaries are near metabolic balance and that total organic carbon (TOC) export to the coastal ocean is about equal to riverine TOC input (Herrmann et al., 2015; Crosswell et al., 2017). The GOM shares many of these traits, but its TOC input is low due to its small catchment area (Najjar et al., 2018).
Atlantic Coast Tidal Wetlands
Despite some similarity in vegetation community composition (e.g., estuarine emergent Spartina spp., dominant in saline habitats), Atlantic coast tidal marshes are extensive and topographically varied in structure, from the more patchy, organic-rich GOM and MAB soils to the extensive, mineral-rich plains of the SAB. Biomass stocks of the dominant plant species, Spartina alterniflora, show a decrease with latitude (Kirwan et al., 2009), with the notably productive SAB marshes (Gallagher et al., 1980; Schubauer and Hopkinson 1984) exporting large amounts of marsh grass–derived organic matter and CO2 into the estuaries and nearshore ocean where respiration and degassing occur (Jiang et al., 2008; Wang and Cai 2004). Soil carbon burial is not commensurate with productivity, as increased organic matter decomposition (Kirwan and Blum 2011) may negate any latitudinal productivity gradients. More important than latitudinal patterns for carbon flux accounting are within-watershed patterns of marsh elevation (i.e., low marsh versus high marsh), tidal range (e.g., microtidal eastern Florida versus extreme macrotidal Bay of Fundy), and salinity regimes. Freshwater tidal wetlands (both marsh and forest) make up 21% of tidal wetlands of the eastern United States (Hinson et al., 2017). Localized hotspots for soil carbon stock change also occur along the East Coast because of physical drivers such as sea level rise (Sallenger et al., 2012) and storm-induced erosion (Cahoon 2006). Estimated net ecosystem exchange (NEE) of atmospheric CO2 from chamber and eddy covariance systems illustrates that vertical fluxes dominate carbon inputs to many East Coast tidal wetlands (Forbrich and Giblin 2015; Kathilankal et al., 2008). Much of this NEE is exported to ocean subsystems in particulate and dissolved forms, with lateral exports of DIC and DOC fluxes representing as much as 80% of annual carbon inputs (Wang and Cai 2004; Wang et al., 2016). Further, the role of groundwater flows in driving carbon fluxes, as well as nutrient fluxes that alter estuarine processes, is varied and poorly understood (Kroeger and Charette 2008; Moore 1996).
15.3.2 Gulf of Mexico Estuaries and Tidal Wetlands
Variability of Gulf of Mexico (GMx) estuaries is due, in part, to the variable forcing at their boundaries, including groundwater (dominating the Mexican coastline), rivers (dominating the U.S. coastline), wind, bathymetry, and ocean currents (e.g., the Loop Current). Gulf of Mexico tidal wetlands share many species but notably are experiencing enhanced mangrove encroachment and land subsidence.
Gulf of Mexico Estuaries
Estuarine GMx environments are microtidal with winds and river flows exerting strong control on water levels. On the extensive subtidal carbonate benthos, extensive seagrass meadows (e.g., Thalassia) persist and are known to recover rapidly from disturbance (e.g., Thorhaug et al., 2017). There is a paucity of data on air-water CO2 flux in GMx estuaries. However, the lower-river portion of the two largest rivers, the Mississippi and the Atchafalaya, are strong sources of CO2 to the atmosphere because the partial pressure of CO2 (pCO2) ranges from about 1,000 microatmospheres (μatm: a unit of pressure defined as 101,325 Pascals or 1.01325 bar) in winter to about 2,200 μatm in summer, but some large bays (e.g., Terrebonne Bay) have substantially lower pCO2 (Huang et al., 2015). In comparison, despite relatively low pCO2 (about 500 µatm), a semi-arid lagoonal estuary in northwestern GMx has a CO2 efflux of 149 ± 40 grams of carbon (g C) per m2 per year due to windy conditions all year long (Yao and Hu 2017), an amount comparable to other lagoonal estuaries in the world (Laruelle et al., 2014). A strong climatic gradient from northeast to southwest along the northwestern GMx coast leads to riverine freshwater export decreasing by a factor of two (Montagna et al., 2009), with large interannual variability. This hydrological variability exerts strong control on estuarine CO2 fluxes in this region.
Gulf of Mexico Tidal Wetlands
As of 2017, 52% of conterminous U.S. tidal wetlands are located within GMx, with Louisiana alone containing 40% of all the saltwater wetlands in the United States (Dahl 2011; Edwards and Proffitt 2003). While the GMx U.S. coastline is dominated by emergent marsh vegetation and the Mexican coastline is dominated by mangrove vegetation (see Table 15.1), a wide range of salinity and geomorphic conditions promote structural diversity throughout GMx from tidal freshwater forests to floating peatlands to brackish and saline marshes. For the past two decades, other coastlines have been relatively stable in their tidal wetland extent but GMx is experiencing rapid transitions. Though there is active delta building at the Atchafalaya River outflow, tidal wetland conversion to open water (i.e., wetland loss) is common in GMx as a result of land subsidence, coastal storms, sea level rise, nutrient enrichment, and a lack of sediment delivery to compensate for ongoing compaction. The fate of wetland soil carbon following erosion or conversion to open water is poorly understood but important for conducting carbon accounting, particularly in GMx (DeLaune and White 2011; Lane et al., 2016). Climate shifts are also accelerating changes in wetland cover (Gabler et al., 2017), including mangrove encroachment on salt marshes in Texas, Louisiana, and Florida (Krauss et al., 2011; Saintilan et al., 2014).
Mangroves extend all the way around GMx, with 80% of the total distribution of North American mangroves on the Mexican coastline (50% of which grow on the Campeche, Yucatán, and Quintana Roo coasts). Mangrove carbon sequestration rates can range from 0 to 1,000 g C per m2 per year, primarily a result of biomass responses to disturbance status and hydrogeomorphic characteristics of the landscape setting (Adame et al., 2013; Breithaupt et al., 2014; Ezcurra et al., 2016; Marchio et al., 2016). Regular tidal flushing and allochthonous input from river and marine sediments generally provide more favorable conditions for above- and belowground productivity. The belowground components of mangrove forests, such as coarse woody debris, soil, and pneumatophores (i.e., aerial roots), can contribute between 45% and 65% of the total ecosystem respiration (Troxler et al., 2015). Mangroves are similar to all tidal wetlands in that soil carbon pools dominate ecosystem carbon stocks, and carbon burial is an important long-term fate of fixed carbon. For example, despite their short stature, dwarf mangroves may generate greater annual increases in belowground carbon pools than might taller mangroves (Adame et al., 2013; Osland et al., 2012).
Coupled stressors from both human and natural drivers, such as groundwater extraction and sea level rise, currently are altering subtropical tidal wetlands. Soil organic carbon (SOC) stocks face increased rates of mineralization and peat collapse with saline intrusion (Neubauer et al., 2013). Still, total carbon stocks may increase as a result of trends in mangrove expansion into salt marsh habitat (Cavanaugh et al., 2014; Doughty et al., 2015; Krauss et al., 2011; Bianchi et al., 2013). This pattern of expansion is expected to continue with current trends in climate change (e.g., the changes in frequency and intensity of hurricanes and freeze events) and with increasing rates of sea level rise (Barr et al., 2012; Lagomasino et al., 2014; Meeder and Parkinson 2017; Dessu et al., 2018). Dwarf and basin mangroves, which generally have shorter canopies, are most affected by freezing temperatures, while hurricane damage has the strongest impact on fringing mangrove forests along the coasts (Zhang et al., 2016). Freeze and cold events drive the poleward advancement of mangroves along the eastern coast of Florida and GMx (Cavanaugh et al., 2014; Giri et al., 2011; Saintilan et al., 2014). Though mangroves in these regions may not currently extend past their historical range limits (Giri and Long 2014), the expansion and contraction of the mangrove forest clearly is documented in field and remotely sensed map products.
15.3.3 Pacific Coast Estuaries and Tidal Wetlands
The Pacific (west) coast of North America is seismically active with subduction zones that create steep topography and narrow continental shelves. As such, seasonal coastal winds drive upwelling and downwelling events that can shape biogeochemical cycling along the Pacific continental margin in estuarine waters and tidal wetlands. A more descriptive approach herein reflects the limited representation of Pacific Coast information presented in Appendix 15A as compared with that for the Atlantic and GMx coastlines.
Pacific Coast Estuaries
Estuaries of the Pacific Coast differ from other North American estuaries in that their carbon cycle dynamics tend to be dominated by ocean-sourced rather than river-borne drivers, predisposing many Pacific Coast estuaries and coastal environments to hypoxia and acidified conditions, largely as a result of natural processes (e.g., Chan et al., 2016, 2017; Feely et al., 2010, 2012; Hales et al., 2016). From the Gulf of Alaska south through Puget Sound, glacially formed estuaries have sills that restrict circulation between estuaries and coastal waters, further predisposing deep estuarine waters to hypoxic or anoxic conditions that form in the deep water of these estuaries. Interannual-to-decadal, basin-scale, ocean-climate oscillations such as the Pacific Decadal Oscillation and El Niño Southern Oscillation drive variations in rainfall along the Pacific Coast, which, in turn, controls material export from land to estuaries and subsequently to the coastal ocean. These oscillating climate drivers, as well as stochastic events such as large marine heatwaves, drive interannual variability in physical and biogeochemical dynamics along the Pacific Coast, with significant effects on estuarine carbon cycle and ecosystem processes (Di Lorenzo and Mantua 2016).
Within spatially large marine ecosystems (LMEs) on the Pacific Coast—Gulf of Alaska, California Current, Gulf of California, and PacificCentralAmerican Coastal LMEs (lme.noaa.gov)—estuaries represent either globally significant large river systems, such as the Fraser, Columbia, San Joaquin/Sacramento, and Colorado rivers or one of many “small mountainous rivers” (SMRs) with steep watershed terrain and limited continental shelves for delta development. From the Southern California Bight (SCB) south to Panama, lagoons also represent a significant fraction of the semi-enclosed, saline-to-brackish water bodies along the Pacific Coast. Lagoons typically have episodic connection to adjacent coastal ocean areas and lack substantial freshwater input, distinguishing them from estuaries. However, despite the strong along-coast gradients in rainfall and terrestrial input to Pacific Coast lagoons and estuaries, oceanic sources of nutrients and carbon, particularly those delivered via upwelling, play an important or dominant role in carbon cycle dynamics in all systems studied (Camacho-Ibar et al., 2003; Davis et al., 2014; Hernández-Ayón et al., 2007; Steinberg et al., 2010).
Terrestrial inputs to Pacific Coast estuaries vary substantially along the steep rainfall gradient from very wet conditions in the north to arid conditions in southern and Baja California, with precipitation increasing again from central Mexico through Panama. The Global NEWS 2 model estimated terrestrial TOC inputs are approximately 8.5 teragrams of carbon (Tg C) per year to the Gulf of Alaska through northern California, 0.7 Tg C per year to southern and Baja California and the Gulf of California, and 2.8 Tg C per year to Mexico south of Baja California and Central America (Mayorga et al., 2010). The SMRs representing a significant portion of these inputs are similar to the Mississippi River in delivering their freshwater, nutrient, and organic carbon loads directly to the coastal ocean or larger estuarine water bodies such as Puget Sound or the Strait of Georgia (Johannessen et al., 2003; Wheatcroft et al., 2010).
Phytoplankton productivity estimates across Pacific Coast estuaries from San Francisco Bay to British Columbia reflect an order of magnitude variation in median annual primary production rates, from about 50 g C per m2 per year in the Columbia River estuary to 455 to 609 g C per m2 per year in the Indian Arm fjord near Vancouver, British Columbia (Cloern et al., 2014). The role of riverborne nutrients is exemplified by the total water column primary production estimate for the Columbia River estuary at 0.030 Tg C per year (Lara-Lara et al., 1990). An air-sea CO2 exchange study on the Columbia River estuary estimated that the net annual emission is quite small at 12 g C per m2 per year (Evans et al., 2012). SCB estuaries are also highly productive but most likely act as sources of CO2 to the atmosphere and net exporters of dissolved inorganic and organic carbon to the coastal ocean owing to input and decomposition of allochthonous carbon from surrounding land areas. All recent studies from lagoons and estuaries in the San Diego area report estuarine pCO2 levels consistently greater than atmospheric levels (Davidson 2015; Paulsen et al., 2017; see also Southern California Coastal Ocean Observing System: sccoos.org/data/oa). Carbon cycling in lagoons with little or no riverine input is likely to be dominated by upwelling, as in San Quintín Bay, Baja California. Most of San Quintín Bay (85%) acts as a source of CO2 to the atmosphere (131 g C per m2 per year) due to the inflow and outgassing of CO2-rich upwelled waters from the adjacent ocean. The remaining 15%, composed of Zostera marina seagrass beds, shows net uptake of CO2 and bicarbonate (HCO3–), with pCO2 below atmospheric equilibrium, resulting in a net CO2 sink of 26 g C per m2 per year (Camacho-Ibar et al., 2003; Hernández-Ayón et al., 2007; Munoz-Anderson et al., 2015; Reimer et al., 2013; Ribas-Ribas et al., 2011). Whereas this Mediterranean climate bay was net autotrophic during the upwelling season in previous decades, it now appears to be net heterotrophic due to import of labile phytoplanktonic carbon generated in the adjacent ocean during upwelling (Camacho-Ibar et al., 2003). This transition illustrates the potential sensitivity of estuarine, bay, and lagoonal net ecosystem production (NEP) to changes in upwelling intensity and persistence, highlighting the vulnerability to effects of ocean warming or changing coastal stratification on ecosystem metabolism and carbon balance.
Lateral transfers of carbon from estuaries to the coastal ocean are poorly constrained by observations because of the difficulty and expense of making sufficient direct observations to measure this important lateral transfer. Many gaps remain in the understanding of the carbon cycle of Pacific Coast estuaries and lagoons, despite sporadic observations over the last several decades. For example, no systematic information on carbon burial is available and seagrass extent is likely undermapped (CEC 2016). With few exceptions, long-term monitoring time series are inadequate to track changes in terrestrial carbon inputs, primary production, air-sea CO2 exchange, carbon burial in sediments, and carbon transfers to the coastal ocean that can be expected to result from climate and human-caused environmental changes (Boyer et al., 2006; Canuel et al., 2012). Implementing long-term observations of carbon, oxygen, and nutrient biogeochemistry, along with metrics of ecological response and health, in Pacific Coast estuaries is a priority (Alin et al., 2015).
Pacific Coast Tidal Wetlands
The Pacific Coast is dominated by rocky headlands, broad sand dune complexes, sand beaches, and spits (i.e., sandbars). The area of Pacific Coast tidal wetlands is roughly 628 km2 in the United States (NOAA 2015) and at least 2,522 km2 in Mexico, predominantly as mangroves (Valderrama-Landeros et al., 2017), perhaps more if shallow water habitats are included (Contreras-Espinosa and Warner 2004). While small but iconic “low-flow” estuaries are distributed sparsely along the coast (e.g., Elkhorn Slough and Tomales Bay), areas of expansive estuarine wetlands are limited to the larger coastal estuaries, where major rivers enter the sea and where embayments are sheltered by sandbars or headlands (e.g., Coos Bay, Humboldt Bay, and San Diego Bay). San Francisco Bay, which supports the largest extent of coastal wetlands along the Pacific Coast of North America, is a tectonic estuary—a down-dropped graben (i.e., trench) located between parallel north-south trending faults. In Mexico, coastal wetlands are found in association with large barrier-island lagoon complexes where wave energy is reduced by headlands, offshore islands, or the Baja California peninsula, as well as along the Gulf of Tehuantepec, where the continental shelf widens and the winds are intense and offshore (northerly), originating in the Gulf of Campeche across the Isthmus of Tehuantepec. Assuming that published studies of soil carbon accumulation (79 to 300 g C per m2 per year (Ezcurra et al., 2016) are broadly representative of U.S. and Mexico coastlines, average estimates of soil carbon sequestration by Pacific estuarine wetlands sum to 0.05 Tg C per year for the United States and 2.67 Tg C per year for Mexico.
Although U.S. Atlantic and GMx coastlines are known to support more organic-rich sediments, rates of carbon burial in tidal wetlands on the Pacific Coast tend to be commensurately high due to high rates of volume gain through sediment accretion. Previous studies have reported accretion rates of 0.20 to 1.7 cm per year in natural marshes along the Pacific Coast of North America (Callaway et al., 2012; Thom 1992; Watson 2004), with many values at the higher end of this range. High rates of sediment accretion are a function of the active Pacific Coast margin, because Pacific coastal watersheds tend to have high relief and support elevated erosion rates while providing limited opportunity for deposition of sediments along lowland floodplains (Walling and Webb 1983). This circumstance leads to high water column–suspended sediment concentrations, often exacerbated by anthropogenic land-use activities, such as agriculture, grazing, logging, and development (Meybeck 2003). Although not ubiquitous due to landscape changes (e.g., Skagit River), high rates of sediment accretion are common and known to promote high carbon burial rates when allochthonous organic carbon derived from upland sources is a sediment constituent (Ember et al., 1987). Additionally, organic carbon produced in situ is more quickly buried in the sediment anoxic zone in high-accumulation environments (Watson 2004).
15.3.4 High-Latitude (Alaskan, Canadian, and Arctic) Estuaries and Tidal Wetlands
High-latitude estuaries (boreal and Arctic) are the youngest estuaries (<1,000 years) but the most subject to coastal erosion and hydrological carbon export from thawing permafrost during the current warming climate. Terrigenous inputs of silt and organic carbon are estimated as dominant sources of carbon flux, but inadequate mapping and measurements limit current estimates of carbon fluxes in high-latitude estuaries and tidal wetlands.
High-Latitude (Arctic) Estuaries
Salinity gradients are a defining feature of the estuarine zones of the Arctic Ocean (McClelland et al., 2012). Further, nearshore ice conditions are changing, erosion of coastlines is increasing, and the duration and intensity of estuarine and ocean acidification events are increasing (Fabry et al., 2009), as also discussed in Ch. 16: Coastal Ocean and Continental Shelves and Ch. 17: Biogeochemical Effects of Rising Atmospheric Carbon Dioxide. Lagoons in the Alaskan Beaufort Sea, bounded by barrier islands to the north and Alaska’s Arctic slope to the south, span over 50% of the coast. These lagoons link marine and terrestrial ecosystems and support productive biological communities that provide valuable habitat and feeding grounds for many ecologically and culturally important species. Beaufort Sea lagoons are icebound for approximately 9 months of the year; therefore, the brief summer open-water period is an especially important time for resident animals to build energy reserves (i.e., necessary for spawning and surviving winter months) and for migratory animals to feed in preparation for fall migrations. Recent dramatic declines in ice extent have allowed wave heights to reach unprecedented levels as fetch has increased (AMAP 2011).
These studies highlight the climate linkages along coastal margins of the Arctic, especially how changes in sea ice extent can affect terrestrial processes (Bhatt et al., 2010), controlling coastal erosion and the transport of carbon, water, and nutrients to nearshore estuarine environments (Pickart et al., 2013). Nearshore estuarine environments in the Arctic are critical to a vibrant coastal fishery (von Biela et al., 2012) and also serve as habitat for hundreds of thousands of birds representing over 157 species that breed and raise their young over the short summer period (Brown 2006).
High-Latitude (Arctic) Tidal Wetlands
High-latitude ecosystem carbon flux measurements tend to focus on abundant inland peatlands (see Ch. 11: Arctic and Boreal Carbon and Ch. 13: Terrestrial Wetlands), and thus less is known about Arctic and subarctic tidal marshes. However, due to high sedimentation rates, Arctic estuarine wetlands are estimated to sequester carbon at rates up to tenfold higher per area than many other wetlands (Bridgham et al., 2006). In a North American survey of published literature, Chmura et al. (2003) accounted for soil carbon stock only to 50 cm in depth, but some brackish marshes, especially in seismically active regions, have much deeper organic sediments. The Hudson Bay Lowlands tidal marshes are a notably understudied region where soil carbon stocks in the nontidal component alone are estimated to contain 20% of the entire North American soil carbon pool (Packalen et al., 2014). Gulf of Alaska marshes are relatively low salinity or freshwater dominated due to the excess of precipitation over evapotranspiration of the Pacific Northwest, as well as the substantial glacial meltwater that characterizes the region. Still, the large impact of melting glaciers, including the Bering and Malaspina piedmont glaciers (each approximating the size of Rhode Island), is expected to contribute to sea level rise locally, as will thawing river deltas, such as the Yukon-Kuskokwim Delta, that are characterized by discontinuous permafrost.
One of the most important coastal Alaskan marsh systems is the Copper River Delta, a critical habitat for migratory birds along the Pacific Flyway, which extends for more than 75 km and inland as much as 20 km in some places along the Gulf of Alaska (Thilenius 1990). Although carbon storage estimates in these marsh locations are lacking, extensive research on the uplifted (and buried) peats by Plafker (1965) indicate alternating events of extreme subsidence and uplift (i.e., yo-yo tectonics). For example, the 1964 earthquake raised the entire delta from 1.8 to 3.4 m (Reimnitz 1966). Current studies on peat cores reveal marsh vegetation interspersed with intertidal muds, freshwater coastal forest, and moss peat, which extends to depths greater than 7 m (Plafker 1965). Whereas geological drivers clearly are the primary control on carbon storage in these marshes, the dynamic relationship with vegetation illustrates biological feedbacks as well (e.g., nutrient redistribution; Marsh et al., 2000). Highly dynamic sedge- and rush-dominated marshes are notably resilient to extensive sediment deposition from the Copper River, further ensuring growth of willows and shrubs and contributing to the woody component of buried peats. Whether the areal extent of these wetlands will expand or decline with tectonic impact and regional sea level rise is not known.