Key Finding 1
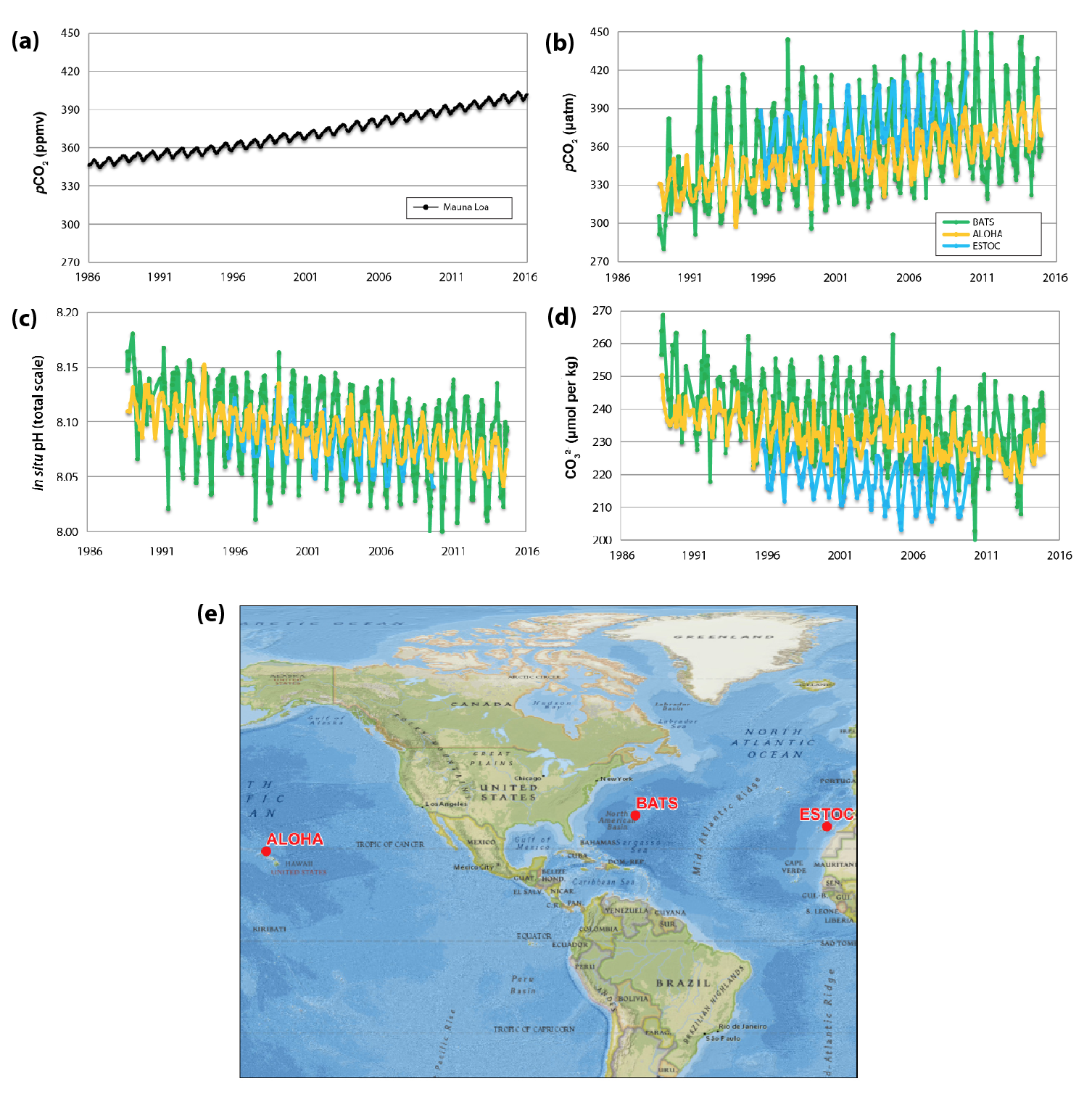
Rising carbon dioxide (CO2) has decreased seawater pH at long-term observing stations around the world, including in the open ocean north of Oahu, Hawai‘i; near Alaska’s Aleutian Islands; on the Gulf of Maine shore; and on Gray’s Reef in the southeastern United States. This ocean acidification process has already affected some marine species and altered fundamental ecosystem processes, and further effects are likely (high confidence, likely).
Description of evidence base
The atmospheric record indicates that both the ocean and land carbon sinks have increased as CO2 has risen (Le Quéré et al., 2016). Modern-day ocean observations have confirmed that seawater pH is decreasing because of atmospheric CO2 uptake (Feely et al., 2004, 2009; Gattuso et al., 2015; Orr et al., 2005). Time-series stations around North America (near Hawai‘i, Alaska, Washington, California, Georgia, and Maine) have documented decreased pH below preindustrial levels for some or all of the annual cycle (Sutton et al., 2016). Effects on marine life and fundamental ecosystem processes or characteristics, including calcification, biodiversity, growth rates, and nitrogen fixation, are reviewed in this chapter; they are documented in detail in Bijma et al. (2013), Bunse et al. (2016), Dupont et al. (2010), Fu et al. (2007, 2012), Hendriks and Duarte (2010), Hendriks et al. (2010), Hofmann et al. (2010), Hutchins et al. (2013), Kroeker et al. (2013), Meyer and Riebesell (2015), Riebesell and Tortell (2011), and Riebesell et al. (2007), among others. Future effects are projected by observational (Pespeni et al., 2013; Wootton et al., 2008), integrative (Boyd et al., 2014), and modeling (Dutkiewicz et al., 2015) studies.
Major uncertainties
In most cases, observed biological effects have not been mechanistically attributed to pH or carbonate and bicarbonate ion concentration changes. Laboratory studies may not perfectly reproduce the responses of organisms in nature, where environments and drivers are more complex and numerous. Genetic, behavioral, and phenotypic plasticity (flexibility) have not been evaluated for most of the species investigated in laboratory studies.
Estimated likelihood of impact or consequence, including short description of basis of estimate
Variation within populations (plasticity) and the existence of many competing environmental drivers could offset the effects of ocean acidification on some marine populations, but to an uncertain extent. Research has demonstrated effects on large groups of marine organisms (e.g., bivalve shellfish and stony corals) unambiguously enough to ascertain that continuing negative impacts to these communities are likely.
Summary sentence or paragraph that integrates the above information
Rising CO2 has decreased seawater pH (very high confidence). This process of ocean acidification has affected some marine species (very high confidence) and altered fundamental ecosystem processes (high confidence), with further effects likely (high confidence). Continuing impacts are probable, but plasticity and the existence of other environmental drivers could offset the effects of ocean acidification on some marine populations to an uncertain extent.
Key Finding 2
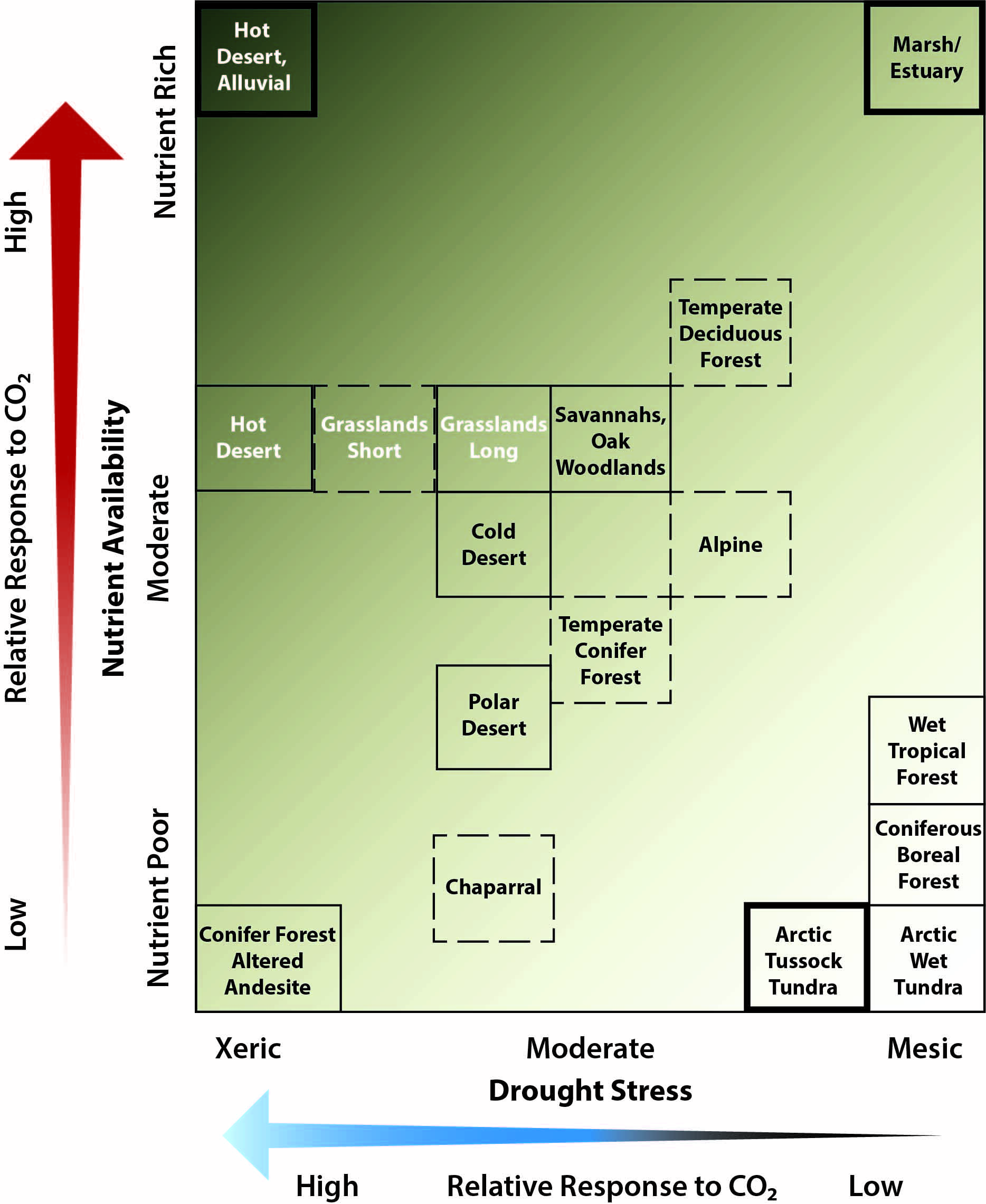
While atmospheric CO2 rises at approximately the same rate all over the globe, its non-climate effects on land vary depending on climate and dominant species. In terrestrial ecosystems, rising atmospheric CO2 concentrations are expected to increase plant photosynthesis, growth, and water-use efficiency, though these effects are reduced when nutrients, drought, or other factors limit plant growth (very high confidence, very likely). Rising CO2 would likely change carbon storage and influence terrestrial hydrology and biogeochemical cycling, but concomitant effects on vegetation composition and nutrient feedbacks are challenging to predict, making decadal forecasts uncertain.
Description of evidence base
Research definitively shows that the bodies of marine and terrestrial organisms have incorporated CO2 released from the burning of fossil fuels, based on the change in isotope ratios within their biological material (Fraile et al., 2016; Hilton et al., 2006; Suess 1955).
On land, the historical record of the impact of rising CO2 is more complex. Physiological theory suggests that, as CO2 rises, photosynthesis should increase. Using preserved plant specimens, isotopomer analysis appears to support this physiological prediction (Ehlers et al., 2015), though this is a novel technique. The effects of rising CO2 on tree biomass over multiple decades may be inferred from tree-ring records, but they provide mixed results (Andreu-Hayles et al., 2011; Cole et al., 2009; Knapp and Soulé 2011; Koutavas 2013). Studies from a wide range of forest types across broad geographic regions have observed changes in the ratio of the 13C isotope to the 12C isotope (δ13C), observations which imply trees have experienced increased water-use efficiency as CO2 has risen over the last two centuries, but growth was not clearly stimulated by rising CO2 (Peñuelas et al., 2011).
Rising CO2 tends to make plants close their stomata and thus use water more efficiently. The primary enzyme responsible for CO2 uptake, ribulose-1,5-bisphosphate carboxylase-oxygenase (RUBISCO), accounts for a substantial portion of every plant’s nitrogen requirement. As CO2 rises, less RUBISCO is required for the same carbon gain, so plants become more efficient in nutrient use. These physiological effects play out differently in various types of plants and under diverse environmental conditions. Plants that lack a CO2 concentration mechanism and pass a 3-carbon sugar molecule into the Benson-Calvin cycle (C3 plants) are more likely to show an instantaneous photosynthetic response than plants with a CO2 concentration mechanism like C4 plants (that pass a 4-carbon sugar molecule to the Benson-Calvin cycle) or those that use crassulacean acid metabolism (CAM).
Twenty years of CO2 enrichment experiments have shown that elevated CO2 enhances photosynthetic carbon gain over the long term for certain ecosystem types but only over the short term for others (Leakey et al., 2009; Leuzinger et al., 2011; Norby and Zak 2011). Plant communities dominated by trees and grasses generally have shown greater stimulation of photosynthetic carbon uptake compared to that of legumes, shrubs, and nonleguminous C3 crops (Ainsworth and Rogers 2007).
Net primary production (NPP) is calculated as either the balance between carbon gained through photosynthesis and lost through respiration or the sum of all growth over a year. NPP is enhanced by ~23% across a broad range of early successional forests in response to elevated CO2 (Norby et al., 2005). These results are likely not indicative of all forests, and smaller responses have been observed in the limited number of studies carried out in old-growth temperate, boreal, and tropical forests (Hickler et al., 2008; Körner et al., 2005). Also clear is that the temporal pattern of NPP responses to elevated CO2 differs among forests. For example, McCarthy et al. (2010) reported that NPP in coniferous forests was enhanced by 22% to 30% and sustained over 10 years of exposure to 550 parts per million (ppm) of CO2. In contrast, Norby et al. (2010) found that NPP was significantly enhanced for 6 years in hardwood forest plots exposed to 550 ppm CO2 (compared with plots under current ambient CO2), after which time the enhancement of NPP under elevated CO2 declined from 24% to 9%.
Plants balance carbon gain and water loss. Stomatal conductance is depressed at elevated CO2, so plants may reduce water loss without reducing carbon gain. This physiological effect has been observed at the leaf and canopy scales (Keenan et al., 2013; Leakey et al., 2009; Peñuelas et al., 2011) and represents the major mechanism leading to observations of decreased canopy evapotranspiration under elevated CO2. For the hydrological cycle, this mechanism results in increased soil moisture. Even plants with CO2 concentration mechanisms (i.e., C4 and CAM plants) may experience increased water-use efficiency without any direct stimulation in photosynthesis (Leakey et al., 2009). Under drought conditions, elevated CO2 may not directly stimulate photosynthesis in C4 plants but can indirectly increase carbon gain by increasing water-use efficiency.
Physiological theory and experimental evidence indicate that rising CO2 increases the photosynthetic temperature optimum (Long 1991) because of the decreasing relative solubility of CO2 versus oxygen at higher temperatures (Jordan and Ogren 1984). These results imply that biomes that experience high temperatures may experience disproportionately enhanced photosynthesis and growth. Interannual variation in the increased growth of Lobolly pine trees was disproportionately enhanced by experimentally elevated CO2 in warmer years (Moore et al., 2006).
Plant growth is not limited by CO2 alone (Körner 2015). If, for example, another environmental factor limits growth, then experimentally increasing CO2 has reduced effects on photosynthesis and growth (Ainsworth and Rogers 2007). This outcome is called “sink limitation.” Research suggests that nitrogen limitation may be one mechanism leading to declining NPP responses to elevated CO2 in some ecosystems (Norby et al., 2010).
Nitrogen is sequestered in long-lived biomass and soil pools and may not be readily available to plants under some conditions. In this case, nitrogen limitation inhibits increases in plant production associated with elevated CO2, an effect which is referred to as a negative feedback. In systems where nitrogen supply was sufficient, CO2 fertilization effects on NPP persisted (Drake et al., 2011; Finzi et al., 2006). Nevertheless, elevated CO2 also increases photosynthetic nitrogen-use efficiency, defined as the net amount of CO2 assimilated per unit of leaf nitrogen (Ainsworth and Rogers 2007; Bader et al., 2010; Leakey et al., 2009).
Elevated atmospheric CO2 experiments have demonstrated that seed yield can be increased (LaDeau and Clark 2001, 2006). In some crop species, increased seed production was accompanied by reduced quality (Ainsworth et al., 2002), but this was not observed in tree species (Way et al., 2010). Species show different growth responses to rising CO2 (Dawes et al., 2011), and dominant plants may have an advantage with rising CO2 (McDonald et al., 2002; Moore et al., 2006), leading to changes in forest structure.
Major uncertainties
Unclear is whether rising CO2 will lead to larger standing biomass and carbon storage or simply faster cycling of carbon (Norby and Zak 2011). While instantaneous and annual fluxes of carbon are well studied in the Free-Air CO2 Enrichment (FACE) literature, the allocation of carbon to different pools varies between experiments (DeLucia et al., 2005), and enhancement of multidecadal carbon stocks (e.g., woody biomass and soil organic matter) is not well studied (Leuzinger and Hattenschwiler 2013; Norby and Zak 2011). Plant growth is increased by CO2, but gross plant respiration is also stimulated (Leakey et al., 2009). Root growth and the incorporation of organic material below ground are observed in response to elevated CO2 but so too is enhanced soil respiration fueled by releases of carbon from root systems (Drake et al., 2011; Hoosbeek et al., 2007; Jackson et al., 2009; Lagomarsino et al., 2013; Selsted et al., 2012). Increased carbon supply from plants can lead to enhanced activity of soil fauna and more rapid cycling of carbon, rather than increased carbon storage in soils (Phillips et al., 2012; van Groenigen et al., 2011, 2014). Observed changes in soil carbon were small over the timescale of the FACE studies (3 to 16 years), and thus firm conclusions remain elusive (Luo et al., 2011). In general, large effects of rising CO2 on carbon storage in soils are not expected (Schlesinger and Lichter 2001).
The long-term effects of rising CO2 are uncertain because there is only one whole-ecosystem study (i.e., of a salt marsh) that extends to 20 years. Instantaneous physiological responses to CO2 (Farquhar et al., 1980) typically are modified by feedbacks in system-level studies (Leakey et al., 2009; Norby and Zak 2011). Long-term records from tree-ring analyses are limited to reconstructions of aboveground growth. These studies rarely account for changes in carbon allocation strategies (DeLucia et al., 2005; Norby et al., 2010) caused by rising CO2 or changes in nutrient limitation (Finzi et al., 2006; McCarthy et al., 2010; Zhu et al., 2016) or belowground carbon storage (Drake et al., 2011; Phillips et al., 2012; van Groenigen et al., 2014).
Summary sentence or paragraph that integrates the above information
While CO2 is rising globally, there is high confidence that its effects on terrestrial ecosystems will vary across spatial scales because the effects of CO2 on plants vary by species and may be altered by nutrient and water availability. The long-term impacts of rising CO2 on carbon storage in terrestrial ecosystems are uncertain.
Key Finding 3
Consequences of rising atmospheric CO2 are expected to include difficult-to-predict changes in the ecosystem services that terrestrial and oceanic systems provide to humans. For instance, ocean acidification resulting from rising CO2 has decreased the supply of larvae that sustains commercial shellfish production in the northwestern United States. In addition, CO2 fertilization (increases) plus warming (decreases) are changing terrestrial crop yields (high confidence, likely).
Description of evidence base
Commercial oyster larvae in the U.S. Pacific Northwest were significantly damaged by ocean acidification, which caused much higher than usual larval mortality for several years in the mid-2000s (Barton et al., 2015). Harmful impacts on oysters by ocean acidification were well documented (e.g., Kroeker et al., 2013, and references therein). Crop production increased in response to experimentally elevated CO2 (Leakey et al., 2009), accompanied by decreases in seed quality. Decreased protein content has been documented in wheat, barley, rice, potatoes, and soybeans grown at high CO2 (Myers et al., 2014; Taub et al., 2008). Physiological changes also led to increased herbivory in some crops (DeLucia et al., 2012; Dermody et al., 2008). Additional effects are expected for human populations via changes in ocean services, as reviewed in Pörtner et al. (2014). Gattuso et al. (2015) completed a literature review, plus expert judgement assessment, to determine the risk that ocean ecosystem services face from the combined effects of ocean acidification and warming.
Major uncertainties
Uncertainty is related to how rising CO2 may have affected an array of marine and terrestrial harvests and how they may be affected in the future. Evaluating ecosystem services is difficult, and forecasting changes to these services is even more challenging.
Assessment of confidence based on evidence and agreement, including short description of nature of evidence and level of agreement
Very high confidence in the existence and attribution of impacts to increased atmospheric CO2; medium confidence about future projected impacts on ecosystem services.
Estimated likelihood of impact or consequence, including short description of basis of estimate
Studies have already documented impacts to marine and terrestrial harvests. Whether rising CO2 will affect all marine and terrestrial harvests is uncertain.
Summary sentence or paragraph that integrates the above information
Rising CO2 has affected commercial shellfish stocks (very high confidence) and changed crop production yields (very high confidence). Additional consequences expected for human populations include more changes to ecosystem services or changes to benefits that terrestrial and oceanic systems provide to humans (medium confidence). Uncertainty centers around the difficulty of evaluating all exploited species and all ecosystem services and projecting potential future impacts on all of them.
Key Finding 4
Continued persistence of uptake of carbon by the land and ocean is uncertain. Climate and environmental changes create complex feedbacks to the carbon cycle; how these feedbacks modulate future effects of rising CO2 on carbon sinks is unclear. There are several mechanisms that would reduce the ability of land and ocean sinks to continue taking up a large proportion of rising CO2 (very high confidence).
Description of evidence base
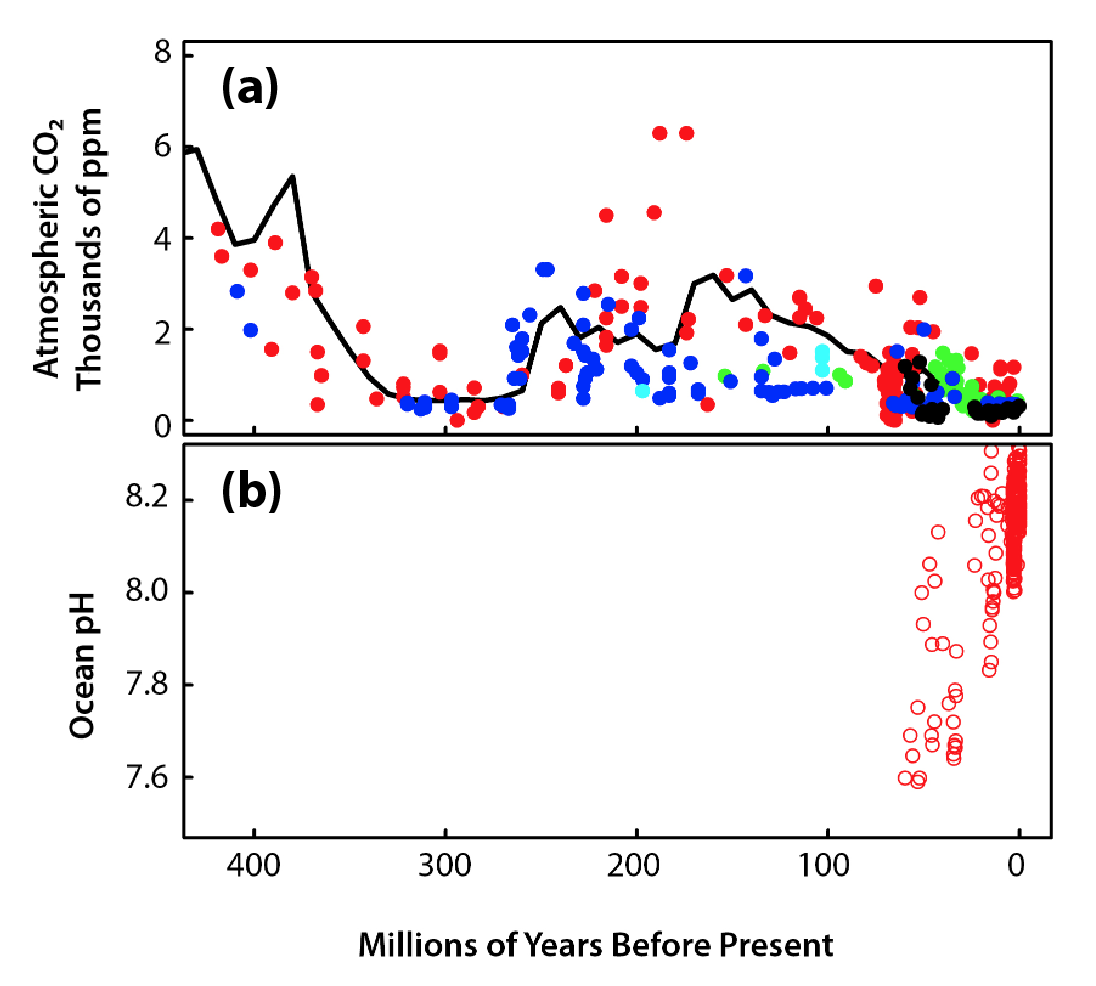
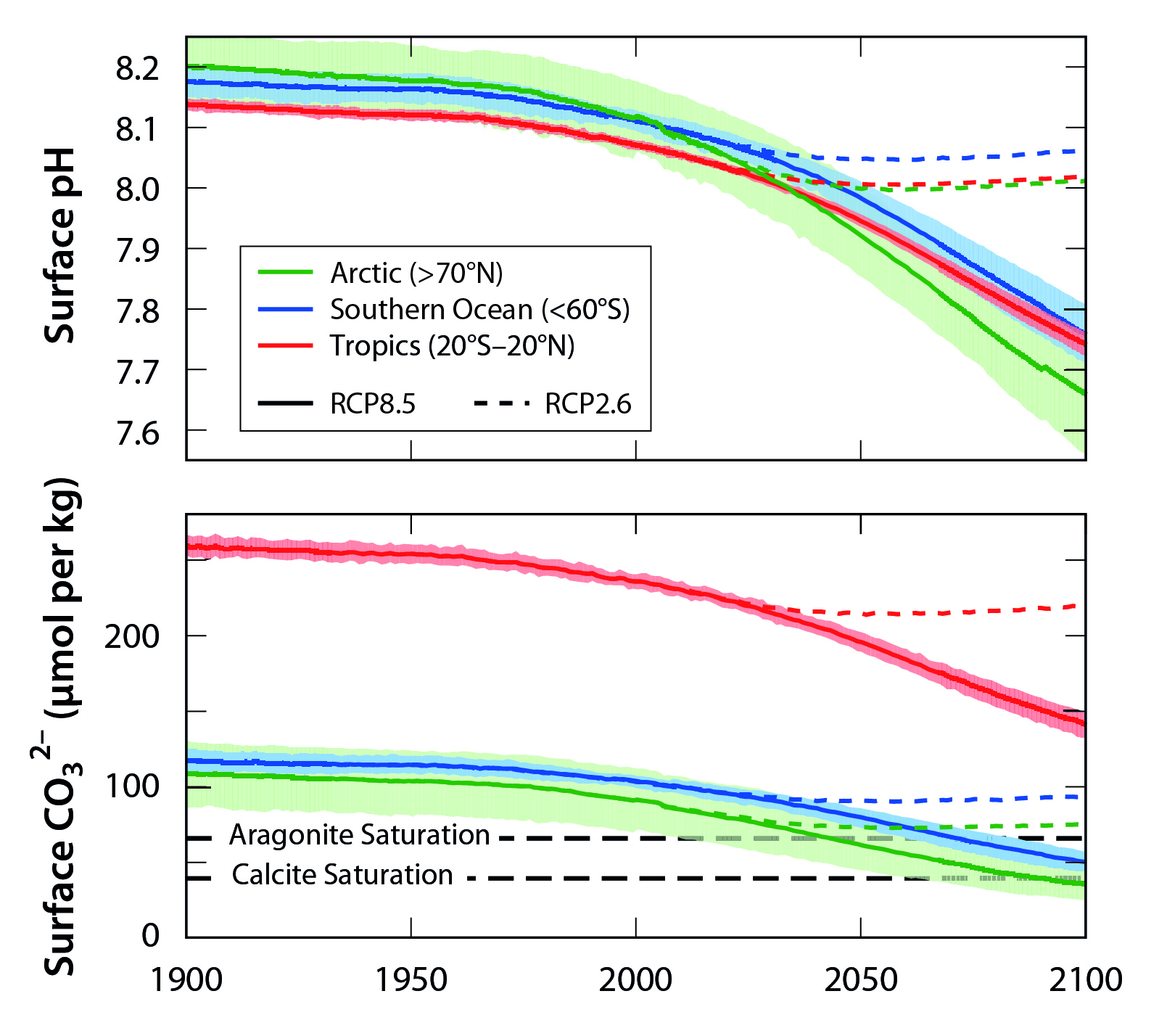
Acidification varies depending on latitude because CO2 solubility depends on temperature, with lower-temperature waters holding more CO2. Polar ecosystems may become undersaturated with calcium carbonate (Ca3O2–) minerals in the near future (Orr et al., 2005; Steinacher et al., 2010) because of the large amount of CO2 already dissolved in cold high-latitude ocean areas. Even though low-latitude ocean areas will not become corrosive to Ca3O2– minerals in the future, conditions will soon surpass the bounds of natural variability (see Figure 17.4). In some places, conditions have already done so (Sutton et al., 2016), exposing low-latitude organisms, such as warm-water coral reefs, to chemical conditions that are considered suboptimal in regard to growth and calcification (Fabricius et al., 2011).
On land, the direct effect of rising CO2 on plant photosynthesis and growth interacts with rising temperature (Gray et al., 2016; Zhu et al., 2016). Rising CO2 increases the photosynthetic temperature optimum (Long 1991) because of the decreasing relative solubility of CO2 versus oxygen at higher temperatures (Jordan and Ogren 1984). Although the sensitivities of photosynthesis, respiration, and decomposition to temperature act on short timescales of decades, chemical weathering sensitivities act over several hundred thousand years and are largely responsible for moderating CO2 levels throughout the geological record. Higher temperatures affect biogeochemical processes through 1) enhanced NPP; 2) faster microbial decomposition of organic matter involving increased emissions of CO2 from microbial respiration in soils; and 3) increased rates of chemical weathering, which consumes CO2 from the atmosphere (Galloway et al., 2014). However, interactions between rising CO2 and temperatures are complicated by nonuniform climate warming patterns, and research shows that this warming can either stimulate or suppress productivity depending on the season and region (Xia et al., 2014). Higher temperatures and drought have been implicated in widespread tree mortality (Breshears et al., 2009; Allen et al., 2010, 2015), and increased aridity in recent years has had a substantially negative effect on forest growth (Allen et al., 2015); these effects are expected to continue (Ficklin and Novick 2017). While some amelioration of physiological stress might be caused by rising CO2 (Ainsworth and Rogers 2007; Blum 2009; Morison et al., 2008), extreme droughts may reduce or eliminate these benefits (Gray et al., 2016). There are very few experiments on tree mortality, but no evidence was found that elevated CO2 reduced drought mortality (Duan et al., 2014).
In the ocean, higher temperatures affect the carbon cycle by decreasing CO2 solubility in seawater (Zeebe and Wolf-Gladrow 2001); a warmer ocean will hold less carbon. Also, increased surface ocean stratification from the warmer water will prevent CO2 absorbed by the surface ocean from penetrating into deeper water masses by reducing deep mixing, thereby decreasing overall oceanic carbon uptake and storage (IPCC 2013). In the cryosphere, higher temperatures thaw permafrost and melt ice, processes which release CO2 and methane (CH4) from microbial respiration back into the atmosphere (Schneider von Deimling et al., 2012).
Rising temperatures thus influence the response of the carbon cycle to rising CO2 in diverse and complicated ways, yielding both positive and negative feedbacks to atmospheric CO2 (Deryng et al., 2016; Dieleman et al., 2012; Holding et al., 2015). Overall, higher temperatures tend to release land and ocean carbon into the atmosphere, while rising CO2 is projected to increase land and ocean uptake (Friedlingstein et al., 2006), but magnitudes are variable and uncertain. Earth System Model assessments that include carbon cycle feedbacks to climate change show that the combined effects of environmental change yield an overall increase in CO2 concentrations and thus would likely contribute to more climate warming. The multimodel average CO2 concentration in 2100 is 985 ± 97 ppm, compared to a concentration of 936 ppm in models lacking carbon cycle feedbacks (Collins et al., 2013). This feedback is highly uncertain because of its dependence on a variety of factors, and thus studies arrive at large ranges in responses (Blok et al., 2010; Elberling et al., 2013; Hodgkins et al., 2014; McCalley et al., 2014; Schneider von Deimling et al., 2012; Schuur et al., 2009). Temperature also indirectly influences CO2 radiative effects. For example, enhanced evaporation from the ocean in a warmer world yields higher atmospheric water vapor concentrations that further amplify the impact of CO2 on climate warming (Myhre et al., 2013).
Major uncertainties
The source or sink status of coastal zones has been difficult to determine, but evidence points to weakening CO2 release from low-latitude coastal zones and strengthening CO2 uptake from mid- and high-latitude systems, leading to greater release of dissolved inorganic carbon to the ocean (Cai 2011).
The effect of rising CO2 on succession and biodiversity remains poorly understood and quantified and could result in changed ecosystem function and different ecosystem services. This lack of understanding also limits the ability to anticipate recovery from acute disturbances such as storms, fires, disease, or insect outbreaks.
Disentangling the impacts of rising CO2 and other concurrent changes in climate, land use, nutrient cycles, and atmospheric chemistry across all ecosystems probably will require long-term, sustained carbon cycle observations and monitoring of ecosystem and socioeconomic consequences. Long-term observing networks are critical to managing ecosystems sustainably and adaptively (e.g., Schindler and Hilborn 2015), and a focus on data management and interoperability across data platforms would improve understanding of long-term responses to rising CO2 (Ciais et al., 2014). Few experiments on land or in the ocean extend to a decade, and the balance of conclusions from observational studies is not settled.
Summary sentence or paragraph that integrates the above information
Both oceanic and terrestrial ecosystems are influenced by CO2 and a variety of environmental controls, including temperature. The effects of climate and CO2 are likely to interact with each other (i.e., the effect of changing CO2 depends on the climatic conditions). These interactions likely will cause complex feedbacks to climate.