Lajtha, K., V. L. Bailey, K. McFarlane, K. Paustian, D. Bachelet, R. Abramoff, D. Angers, S. A. Billings, D. Cerkowniak, Y. G. Dialynas, A. Finzi, N. H. F. French, S. Frey, N. P. Gurwick, J. Harden, J. M. F. Johnson, K. Johnson, J. Lehmann, S. Liu, B. McConkey, U. Mishra, S. Ollinger, D. Paré, F. Paz Pellat, D. deB. Richter, S. M. Schaeffer, J. Schimel, C. Shaw, J. Tang, K. Todd-Brown, C. Trettin, M. Waldrop, T. Whitman, and K. Wickland, 2018: Chapter 12: Soils. In Second State of the Carbon Cycle Report (SOCCR2): A Sustained Assessment Report [Cavallaro, N., G. Shrestha, R. Birdsey, M. A. Mayes, R. G. Najjar, S. C. Reed, P. Romero-Lankao, and Z. Zhu (eds.)]. U.S. Global Change Research Program, Washington, DC, USA, pp. 469-506, https://doi.org/10.7930/SOCCR2.2018.Ch12
Soils
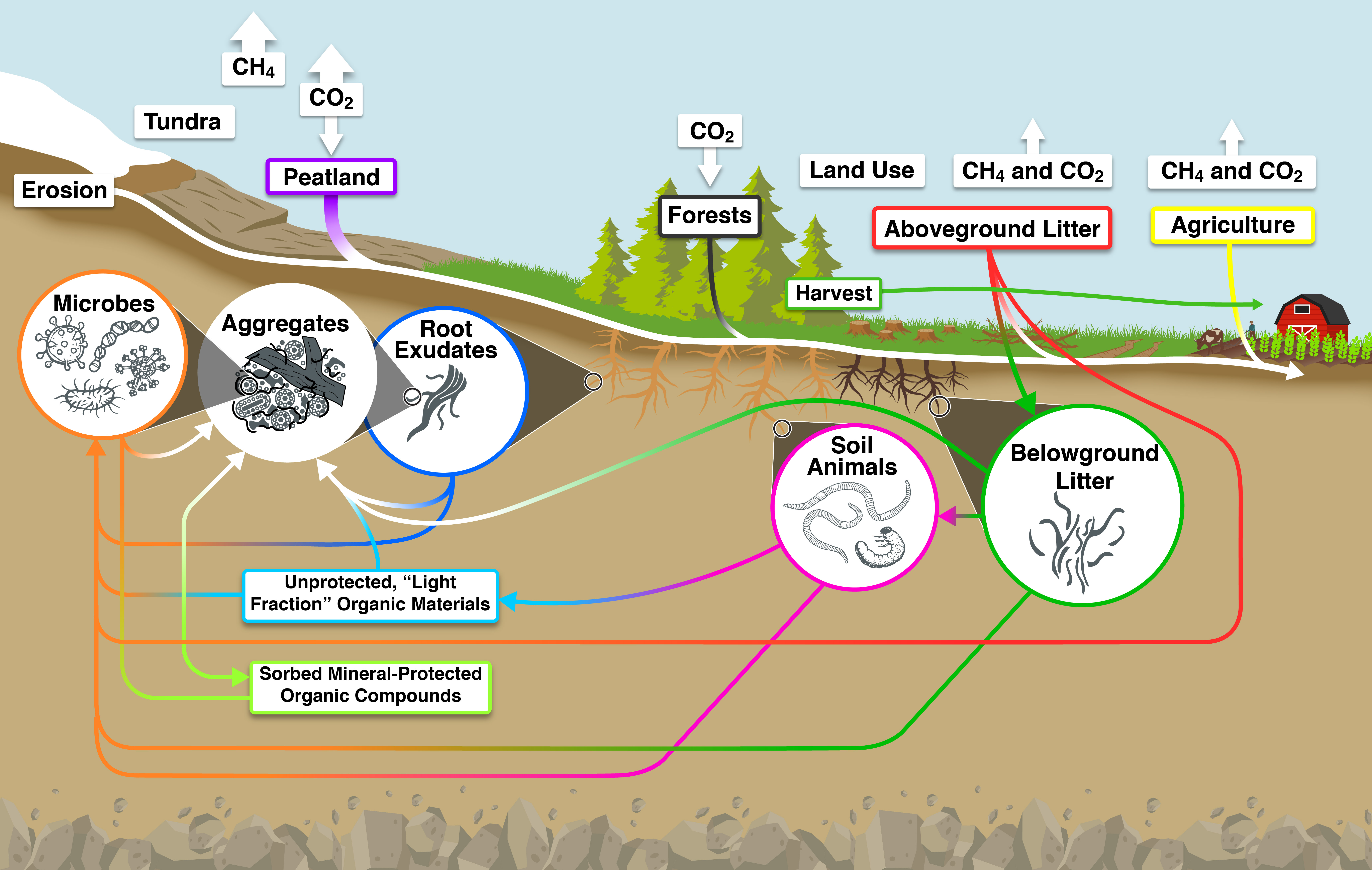
Progress has been made over the last 10 years in understanding specific processes that determine the magnitude and direction of SOC stabilization and destabilization (see Figure 12.1). This new information will not only help explain spatial patterns of SOC in North America, but also will help improve modeling of the large soil carbon pool in Earth System Models (ESMs). Outlined here are the processes that govern overall carbon stocks and fluxes through soils, from inputs through microbial transformations in the bulk soil and rhizosphere, and the protection mechanisms that govern the overall longevity of carbon in soils.
Figure 12.1: Processes Involved in Controlling Fluxes and Stabilization of Soil Carbon
12.2.1 Precipitation
Overriding many soil carbon processes is the complicated role of precipitation and moisture on soil carbon stocks. Precipitation effects on SOC are complicated by the various and often opposing effects of precipitation on the various processes that control carbon stabilization and destabilization. On one hand, where moisture is limiting, increased soil moisture stimulates soil microbial activity, thus increasing soil respiration and destabilization of soil carbon. On the other hand, precipitation has strong effects on both vegetation type and plant production, and thus increases in precipitation in moisture-limited systems generally lead to increases in soil carbon through indirect effects on enhanced plant production, particularly increased root production (Jobbágy and Jackson 2000). In a global analysis (Jobbágy and Jackson 2000) total soil carbon content increased with precipitation and clay content and decreased with temperature. These results match numerous regional studies showing that precipitation in temperate ecosystems has a strong and positive relationship with SOC, likely through effects on total plant biomass, especially belowground biomass (Burke et al., 1989; Liu et al., 2012). Taken together these results suggest a greater response of plant production compared to decomposition from increased precipitation.
Several analyses have noted a wide divergence in estimates of soil carbon stocks from terrestrial biosphere models (Tian et al., 2015; Todd-Brown et al., 2013). Todd-Brown et al. (2013) noted that the parameterization of soil heterotrophic respiration was a significant cause of the discrepancy in model predictions, while Tian et al. (2015) suggested that mechanisms such as changes in the proportion of labile to passive soil carbon pools, as well as sensitivities of respiration to climate, are significant sources of uncertainty in the modeling estimates of soil carbon. Thus, more accurate biome-specific analyses of the effects of precipitation on soil respiration, litter and root production, and vegetation type will be needed to improve soil carbon models.
12.2.2 Plant Litter Inputs
Many factors, including climate regime, atmospheric CO2, land management, soil mineralogy and fertility, and nitrogen deposition strongly influence the structure of the plant community and thus the amount and quality of organic inputs (e.g., litter, wood, and root debris) to the surface of soils (Jandl et al., 2007; McLauchlan 2007; Smith et al., 2007). For example, elevated nitrogen deposition and high soil fertility generally increase plant shoot:root ratios and also decrease concentrations of plant protective compounds such as lignin (Haynes and Gower 1995; Luo and Polle 2009; Pitre et al., 2007). Chemical composition of litter, variably measured as carbon:nitrogen, lignin:nitrogen, or by the presence of complex aromatic compounds, has been shown to influence litter decomposition (Papa et al., 2013; Trofymow et al., 1995; Wardle et al., 2002), with high lignin or aromatic content observed to limit decomposition rates. However, the linkages among litter quantity, litter composition, and SOC stocks are much less clear than would be expected due to other contributing factors. For example, several long-term litter manipulation experiments have shown that increased litter inputs do not always result in increased SOC storage (Lajtha et al., 2014a, 2014b; Mayzelle et al., 2014). Fresh carbon inputs can alter the decomposition of existing SOM because microbes, which play a major role as decomposers in soil ecosystems, will use the new inputs as fuel to decompose existing SOM (Bernal et al., 2016; Crow et al., 2009; Georgiou et al., 2015), resulting in a net decrease in SOC. Site-specific differences in soil mineralogy and microbial physiology also can influence the magnitude of response in SOC concentrations to changes in litter inputs (Geyer et al., 2016; see Section 12.2.3). These kinds of interactions with soil minerals and microbes help to explain why chemical factors, such as lignin content, that are known to control litter decomposition do not always appear to be primary controls on SOC stabilization or destabilization (Rasse et al., 2006; Sulman et al., 2014). There also is evidence that root litter may be preferentially stabilized over shoot-derived litter (Iversen et al., 2008; Kong and Six 2010; Rasse et al., 2005; Russell et al., 2004). Thus, further research is needed to determine how changes in net primary production (NPP), vegetation, and litter quality due to rising atmospheric CO2 concentrations will affect SOC stabilization in the future.
12.2.3 Soil Microbes
Soil microbes, including bacteria, fungi, and archaea, ultimately process all carbon inputs; consequently, microbes are referred to as “the eye of the needle through which all organic materials must pass” (Jenkinson 1977). The organic products and by-products of microbial decomposition, including microbial necromass, can accumulate in soils as SOM, and the chemistry of SOM is distinct from its source material including litter, roots, insect and animal necromass, and wood. The transformation from litter inputs through microbes and into SOM produces inorganic, carbon-containing gases such as CO2 and methane (CH4) through microbial respiration. Because of its important role in carbon transformation, the soil microbial community is key to understanding SOC stocks (Bernal et al., 2016; Guenet et al., 2012), even though the microbial biomass is typically only 1% to 2% of total SOM mass (Xu et al., 2013). Understanding microbial response to microclimate is key to understanding the carbon balance of soils under climate change, because soil balance under changing temperature and moisture is dependent on microbial community and physiological responses to changing temperature and moisture (e.g., Billings and Ballantyne 2013; Yan et al., 2016).
In addition to their direct role mineralizing SOM into inorganic gases, microbes contribute to physical mechanisms of SOC stabilization, indirectly affecting the rate and nature of SOC inputs from plants. A key mechanism of SOC stabilization is protection within soil aggregates (Six et al., 2002), and fungal mycelia and bacterial extracellular polysaccharides are important in forming and stabilizing these aggregates (Aspiras et al., 1971). SOC also is protected by chemical interactions with minerals, particularly silt and clay (Six et al., 2002), and microbes living on minerals may facilitate these interactions by depositing microbially derived carbon directly onto mineral surfaces (Uroz et al., 2015). Microbes can affect plant carbon inputs by regulating plant nutrient supply (Bever et al., 2010; van der Heijden et al., 2006), which affects plant community composition and the timing, mass, and properties of plant inputs of litter and exudates. Thus, although they compose a small fraction of SOC stocks, microbes play a central role in the SOC cycle, affecting inputs, storage, and outputs in diverse ways.
12.2.4 Macrofauna (Food Web)
Soil is home to millions of different organisms, from microorganisms to soil animals (fauna) such as microscopic roundworms (nematodes), tardigrades, rotifers, collembolans, mites, isopods, ants, spiders, and earthworms (Orgiazzi et al., 2015). These fauna exist in food webs containing multiple trophic levels—herbivores that feed directly on the roots of living plants, consumers that feed on living microorganisms associated with dead organic materials, predators that prey on other soil fauna, and plant or animal parasites and pathogens (Coleman and Wall 2015). Through soil bioturbation and feeding on plant roots, organic matter, and their associated microorganisms, soil animals are intimately involved in every step of SOM turnover and soil formation. Sometimes referred to as “ecosystem engineers,” soil animals play a disproportionate role in the carbon cycle relative to their abundance and biomass. Carbon stocks of the soil fauna range from 0.3 to 50 kilograms of carbon per hectare, with desert soils containing the smallest faunal biomass and temperate grassland and tropical rainforest soils the greatest (Fierer et al., 2009). However, across biomes, the biomass of soil fauna typically represents less than 3% of the total biomass of living soil organisms, with soil microorganisms making up the majority. Despite their low biomass relative to soil microbes, soil fauna contribute significantly to carbon cycling through their regulation of microbial activity and through their physical mixing of organic materials and soil. The presence of soil fauna stimulates decomposition, respiration rates (i.e., CO2 flux), and losses of dissolved organic carbon through leaching (de Vries et al., 2013). The positive impact of soil fauna on carbon cycling is attributed to organic matter fragmentation, which increases 1) the surface area available for microbial colonization; 2) the partial digestion of organic materials, enhancing their decomposability; 3) the direct contact of soil microbes with organic matter; and 4) the direct consumption of soil microbes—all impacts which stimulate microbial activity and the release of carbon and nutrients (Coleman and Wall 2015). However, one study found that the activity of earthworms increases carbon stabilization onto minerals to a greater degree than the increase in carbon mineralization, leading to net soil carbon increase (Zhang et al., 2013). Current ecosystem-scale models and ESMs typically overlook the significant effects of soil fauna on the carbon cycle, but guidelines for development of next-generation models call for explicitly incorporating soil food web properties and the responses of soil fauna to land use and climate change (de Vries et al., 2013).
12.2.5 Rhizosphere Interactions
The rhizosphere is defined as an area of soil where microbial activity is stimulated by the presence of roots. A substantial portion of plant biomass is located below ground in the form of roots. Estimates of belowground NPP based on root:shoot ratios assign 30% to 60% of total plant biomass to roots, depending on the biome (Bolinder et al., 2007; Rytter 2001). Regularly shedding sloughed cells and mucilage, roots exude a variety of simple carbon compounds into the soil immediately surrounding them (Hirsch et al., 2013). These root “exudates” comprise primarily organic acids, sugars, and amino acids (Hirsch et al., 2013; Jones 1998). These exudates can interact with minerals by sorption or can liberate organic compounds and nutrients for plant or microbial uptake (Dessureault-Rompre et al., 2007; Keiluweit et al., 2015). In general, the mass of soil in the rhizosphere makes up a smaller fraction (<40%) of total soil than does root-free soil, but it disproportionately affects carbon cycling. For example, microbial biomass, extracellular enzyme activity, decomposition, and mineralization rates are consistently higher in rhizosphere soil compared with those in bulk soil. Fungal hyphae can extend >40 cm away from roots (Finlay and Read 1986), extending the influence of root carbon past the rhizosphere (Zak et al., 1993). Dead root biomass is a substrate source for saprotrophic microbes and detritivores, while living roots are a source of carbon to mycorrhizal fungi.
Mycorrhizal material, shown to be a dominant pathway through which carbon enters the SOM pool, exceeds the input via leaf litter and fine-root turnover (Godbold et al., 2006). Mycorrhizae also may stimulate the decomposition of soil carbon to mine nutrients, paradoxically causing destabilization of soil carbon pools. The effects of mycorrhizae on soil carbon balance are thus complicated by the balance between carbon stabilization effects and soil carbon priming effects (Brzostek et al., 2015). However, recent research (Averill and Hawkes 2016; Averill et al., 2014) demonstrated that ecosystems dominated by plants with symbiotic ectomycorrhizal fungi store more carbon in soils than ecosystems dominated by arbuscular mycorrhizae–associated plants.
12.2.6 Nitrogen Effects on SOM Dynamics
There are substantial interactions between biogeochemical cycles of carbon and nitrogen. Human activities (e.g., fertilizer production, fossil fuel combustion, and industry) have substantially increased nitrogen supply to ecosystems (Vitousek et al., 1997). Global annual nitrogen deposition has increased tenfold over the past 150 years (Lamarque et al., 2005; Yue et al., 2016), although nitrogen deposition has decreased significantly across North America over the last decade due to pollution control. Historic nitrogen loading increased NPP (Elser et al., 2007; LeBauer and Treseder 2008; Xia and Wan 2008), which in turn increased carbon inputs to the forest floor and overall production of plant biomass (Hyvonen et al., 2007; Vitousek et al., 1997). Across biomes, total soil carbon tends to increase with experimental nitrogen addition (Yue et al., 2016), yet this may result less from increases in inputs and more from altering the extent or rates of decomposition (Frey et al., 2014; Liu and Greaver 2010). Microbial decomposition of soil carbon is generally retarded by nitrogen deposition (Hagedorn et al., 2003), but carbon allocation to roots also decreases with nitrogen deposition, limiting new carbon inputs to soil. However, a recent meta-analysis suggested that the reduction in soil carbon respiration, and thus increase in soil carbon stocks resulting from nitrogen deposition, might be equal in magnitude to the amount of additional carbon sequestered by aboveground vegetation (Janssens et al., 2010). Literature surveys suggest that the soil carbon response to anthropogenic nitrogen will fall in the range of 0 to 23 grams of carbon per gram of nitrogen added (Reay et al., 2008), but the uncertainty around this value is very high.
12.2.7 Protection Mechanisms
The extent of carbon protection (i.e., resistance to microbial decomposition) in soil historically has been attributed to litter chemistry, and this remains an element of carbon persistence (Clemente et al., 2011) in organic soils or organic soil horizons that accumulate on the surface of the mineral soil in forests. In recent decades, studies have shown that the controls on carbon stability in mineral soils are more likely dominated by physical and biological factors in the soil environment (Jastrow et al., 2006; Lehmann and Kleber 2015; Lin and Simpson 2016). Physical protection by spatial isolation (i.e., aggregate formation; McCarthy et al., 2008) and chemical associations with soil minerals (i.e., sorption) are both key drivers of carbon persistence in soils. Protection of carbon within soil aggregates (i.e., physical associations between soil minerals and organic compounds) can lead to long-term carbon storage in soils (Jastrow et al., 1996; Six et al., 2004). Compromising the physical structure of aggregates such as by tillage can result in substantial carbon losses because SOC becomes more available physically to decomposition (Navarro-Garcia et al., 2012). Alternatively, carbon may be protected via sorption to soil minerals in which reactive surfaces, including phyllosilicates, oxides, and other minerals, bind carbon molecules via chemical bridges and bonds. The types of compounds sorbed range from discrete chemical compounds (Solomon et al., 2012) to fragments of partially decayed microbial biomass (Courtier-Murias et al., 2013). Mineral-associated carbon stocks can have half-lives ranging from 30 to 4,500 years (Hall et al., 2015a, 2015b; Heckman et al., 2014), yet they can be rendered vulnerable as local environmental conditions change in ways that alter the chemical binding strength, such as changes in precipitation, infiltration, or temperature. In addition, larger-scale processes can serve to protect soil carbon, such as freezing, waterlogging, cryoturbation, or erosion deposition (Kaiser et al., 2007; Grosse et al., 2011; Berhe et al., 2007; Kroetsch et al., 2011).
12.2.8 Losses
Gas Fluxes
Gases including CO2 and CH4 are released from soils as a result of SOM and litter decomposition by soil microbes. Respiration of live roots and their associated mycorrhizal symbionts also release CO2 into the subsurface (Bond-Lamberty et al., 2004; Hanson et al., 2000; Subke et al., 2006; Tang et al., 2005). Globally, approximately 90 to 100 Pg C per year was released to the atmosphere from microbial soil respiration, and the projected rate increase is about 0.1 Pg C per year under a warming climate (Bond-Lamberty and Thomson 2010; Hashimoto et al., 2015). Soil respiration is affected by soil temperature, soil moisture, and organic carbon availability (Davidson and Janssens 2006). Typically, warming increases microbial respiration, while increases in moisture variably affect microbial respiration with maximum CO2 emissions observed under partially saturated conditions. As soils saturate, methanogenesis is likely to emerge as the dominant carbon emission. Other global change factors such as elevated atmospheric CO2 and naturally and anthropogenically altered soil nitrogen status also interactively affect soil respiration in direct and indirect ways (Billings and Ziegler 2008; Zhou et al., 2016). Also observed are vast differences in the amount of gas evolution as a function of landscape heterogeneity, underlying geology and soil type, and vegetative cover, as well as daily and seasonal temporal changes. Consequently, ESMs have not fully used soil respiration data for validation and calibration (Phillips et al., 2016).
Compared with CO2, CH4 has 28 times higher global warming potential over a 100-year time horizon (Saunois et al., 2016). Worldwide biogenic (i.e., associated with plants, animals, and microbes) sources of CH4 emissions, including those from natural ecosystems, agriculture, biomass burning, and landfill waste, are estimated to be 0.33 Pg C per year or 12.4 Pg CO2 equivalent1 (CO2e) per year, including anthropogenic biogenic sources of 7.4 Pg CO2e per year (Tian et al., 2016). The U.S. inventory of greenhouse gases (GHGs) estimated anthropogenic total CH4 emissions of 0.87 Pg CO2e per year in 2015 if the 100-year global warming potential of 28 is used to calculate the CO2 equivalent for CH4, including anthropogenic biogenic sources of 0.42 Pg CO2e per year, mostly from agriculture, landfill, and waste management (U.S. EPA 2017). Methane in North American soils is produced primarily under anaerobic conditions by methanogenic microbes, mostly in freshwater wetlands and rice paddies. However, CH4 emissions are the net balance of both CH4 production and oxidation (i.e., CH4 destruction) by methanotrophic microbes (Tate 2015). The oxidation (i.e., consumption) of CH4 in wetlands is important and may reduce potential CH4 emissions by over 50% (Segarra et al., 2015).
Erosion
Soil erosion mobilizes about 75 Pg of soil each year by water and wind, with most erosion stemming from agricultural lands (Berhe et al., 2007). This accelerated movement of soil has major effects on the carbon cycle, most obviously because erosion physically removes SOC from soil profiles, exposing some fraction to oxidation during transit or upon deposition (Lal 2003). However, the degree to which soil erosion contributes to atmospheric CO2 depends on several additional factors. Erosion can alter SOC mineralization and stabilization at both eroding and depositional sites, for example by burying and partially preserving SOC at the depositional site (Billings et al., 2010; Dialynas et al., 2016). Oxidation of eroded SOC is, therefore, only one component of net SOC change (Van Oost et al., 2012). Stallard (1998) first introduced the concept of new SOC production at an eroding site, a process which can balance the oxidation of eroded SOC (Berhe et al., 2007; Billings et al., 2010; Dialynas et al., 2016; Fang et al., 2006; Harden et al., 1999; Jenerette and Lal 2007; Liu et al., 2003; Quine and Van Oost 2007; Rosenbloom et al., 2006; Smith et al., 2001; Van Oost et al., 2007). Global estimates of the carbon sink strength of erosion and deposition vary widely. Several studies suggest that soil net erosion and deposition may result in a small net carbon sink, perhaps up to about 0.1 Pg C per year (Van Oost et al., 2007), although Berhe et al. (2007) suggest a modern erosion-induced carbon sink strength of about 0.7 to 1 Pg C per year. Wang et al. (2017) estimate a cumulative offset of atmospheric carbon of 78 ± 22 Pg C due to agriculturally enhanced erosion during the period 6000 BC to AD 2015, which represents approximately 37 ± 10% of carbon emissions linked to contemporary anthropogenic land-cover change. Carbon burial rates have increased by a factor of 4.6 since AD 1850, consistent with erosion-induced carbon fluxes occurring disproportionately in recent centuries. Extrapolating globally, Billings et al. (2010) suggest an upper limit of a maximum net global sink of 3.1 Pg C per year (if all eroded carbon were protected from oxidation) and a net source of 1.1 Pg C per year if all eroded carbon were oxidized.
See Full Chapter & References