<b>Bruhwiler</b>, L., A. M. <b>Michalak</b>, R. Birdsey, J. B. Fisher, R. A. Houghton, D. N. Huntzinger, and J. B. Miller, 2018: Chapter 1: Overview of the global carbon cycle. In Second State of the Carbon Cycle Report (SOCCR2): A Sustained Assessment Report [Cavallaro, N., G. Shrestha, R. Birdsey, M. A. Mayes, R. G. Najjar, S. C. Reed, P. Romero-Lankao, and Z. Zhu (eds.)]. U.S. Global Change Research Program, Washington, DC, USA, pp. 42-70, https://doi.org/10.7930/SOCCR2.2018.Ch1.
Overview of the Global Carbon Cycle
Carbon is an essential component of the Earth system. It is fundamental for the existence of life on Earth because of its ability to combine with other important elements, such as oxygen, nitrogen, and phosphorus, and with hydrogen to form the organic molecules that are essential for cellular metabolism and reproduction. Atmospheric carbon in the forms of carbon dioxide (CO2) and methane (CH4) helps regulate the Earth’s climate by “trapping” heat in the atmosphere. This trapping of energy is known as the greenhouse effect, and CO2 and CH4, along with other greenhouse gases (GHGs) such as water vapor and nitrous oxide (N2O), keep the Earth’s climate in a habitable range. Carbon also is of significant socioeconomic importance because the burning of carbon-based fossil fuels is currently the dominant global means of energy production. Production and consumption of coal, oil, and natural gas release CO2, CH4, and other gases to the atmosphere. Considered in this chapter are the global carbon cycle and perturbations to it by human activities, as well as global climate–carbon cycle feedbacks and strategies to control or sequester emissions (see Box 1.1, Why a Global Carbon Cycle Context).
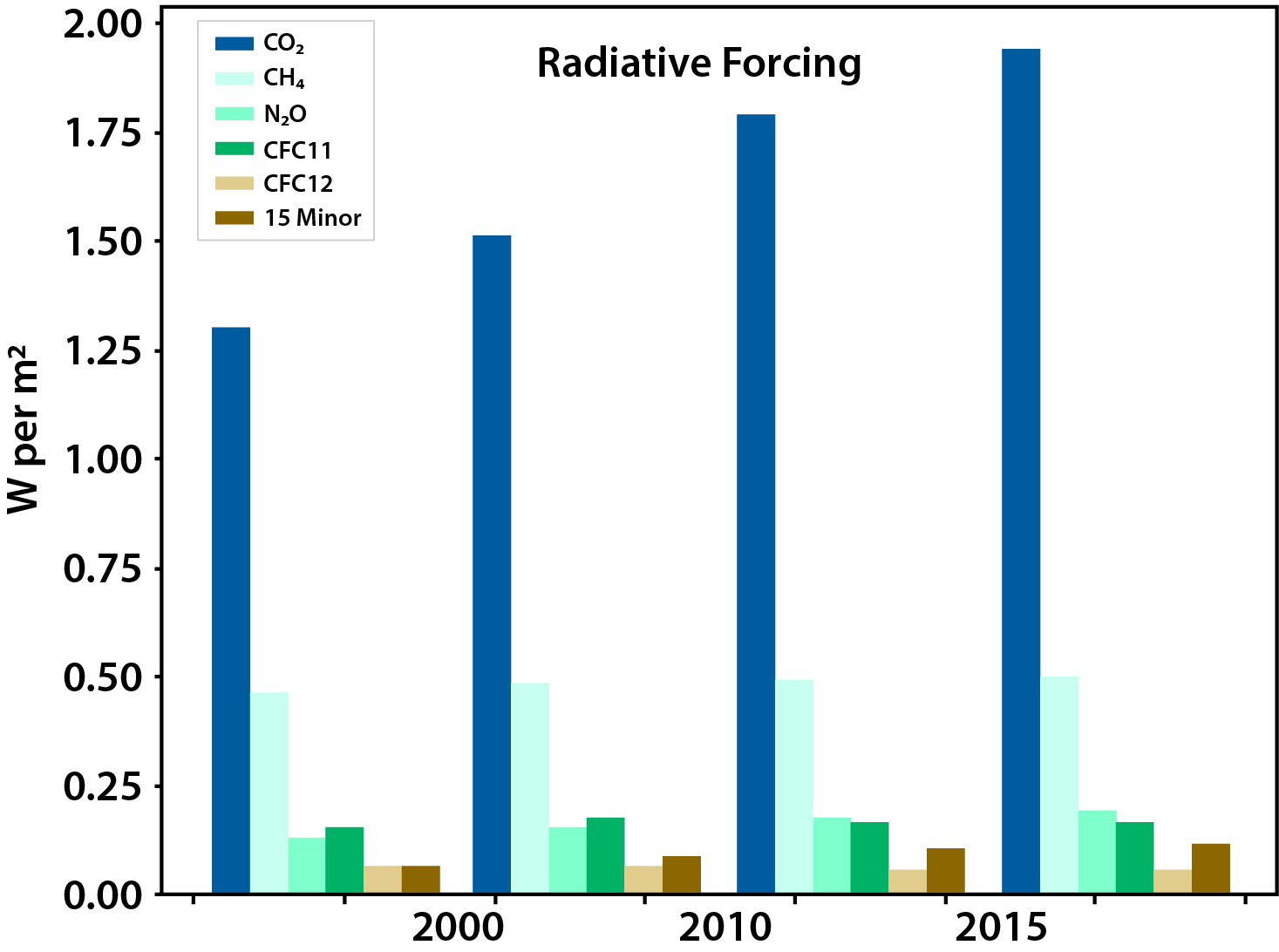
In 2011, the total global radiative anthropogenic forcing (i.e., caused by humans) relative to the year 1750 was 2.8 watts per meter squared (W/m2; Myhre et al., 2013). As of 2017, atmospheric observations of important radiatively active trace species (CO2, CH4, N2O, CFC-11, CFC-12, and 15 minor halogenated gases) suggest that anthropogenic radiative forcing has risen to 3.1 W/m2, an additional 11% (see Figure 1.1).1 The largest portion of this forcing, 2.0 W/m2, is due to CO2, with CH4 accounting for 0.5 W/m2. The global temperature in 2016 relative to the 1880 to 1920 average is greater by 1.25°C in response to this increased radiative forcing (Hansen et al., 2017). Other aspects of the climate system also are changing in response to the increased radiative forcing—the amount, distribution, and timing of rainfall, with extreme hydrological events becoming increasingly frequent, intense, and widespread (Hartmann et al., 2013). These changes may have significant effects on global food production. For example, currently productive regions may not be able to sustain agriculture in the future, especially if water availability becomes limited. Heat stress also can significantly affect agriculture, especially at tropical and subtropical latitudes but also at midlatitudes (Battisti and Naylor 2009). Even though CO2 can result in increased terrestrial plant productivity (i.e., “CO2 fertilization”), the negative impacts of climate change on agriculture are expected to dominate. In the ocean, the decrease in pH of ocean surface water is already about 0.1 pH unit (a decrease in pH of 7.5 to 7.4) since the start of the Industrial Revolution (Bates 2007). This increasing acidification of the ocean, along with water warming and pollution, endangers many marine organisms, including corals, shellfish, and marine plankton. Increasing CH4 emissions can lead to tropospheric ozone formation, with implications for air quality (Fiore et al., 2002). Understanding and predicting future evolution of the global carbon cycle are critical for confronting these issues and, therefore, represent a challenging societal and scientific problem.
Figure 1.1: Radiative Forcing (Relative to 1750) Due to Major Greenhouse Gases (GHGs)
See Full Chapter & References